|

1. The effect of the reactant concentration (CH3COO-)
on the [Fe3(OH)2(CH3COO)6]+ production
A blank solution and test solutions which contain iron(III) and acetate ions in different proportions are prepared on a blister, as summarised in the table below. A simplified drop-based experimental approach is used. The transmittances of the test solutions are measured against the blank using the blue LED,
absorbances are then calculated.
| |
Blank |
Sol. 1 |
Sol. 2 |
Sol. 3 |
Sol. 4 |
|
FeCl3, 0.1 mol/L |
1  |
1  |
1  |
1  |
1  |
|
CH3COONa, 0.1 mol/L |
- |
1  |
2  |
3  |
4  |
|
Deionised water |
8  |
7  |
6  |
5  |
4  |
|
n(Acetate)/n(Fe3+) |
- |
1 |
2 |
3 |
4 |
|
Absorbance |
- |
0.025 |
0.052 |
0.166 |
0.264 |
1  is approximately 36 µl is approximately 36 µl
The results presented in the figure below show the influence of the reactant concentration (CH3COO-) on the [Fe3(OH)2(CH3COO)6]+ production. The reaction between iron(III) and acetate ions is a typical example of an equilibrium reaction. At the stoichiometric proportion between the reactants - n(Acetate)/n(Fe3+) equal to 2 – the reactants only partially convert into products. A significant increase in the absorbance is
observed under other reaction conditions, demonstrating that higher yields are only achieved for an increase in reactant concentration.
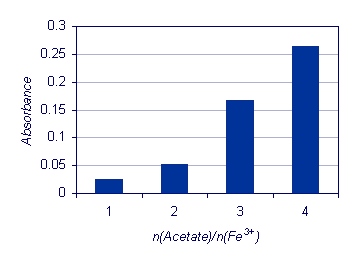
Developed
and prepared
by: Nataša
Gros, University of Ljubljana,
Faculty of Chemistry and Chemical
Technology
|