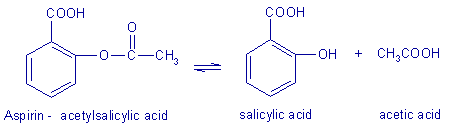|
|
Holes on blister |
1. |
2. |
3. |
4. |
5. |
6. |
7. |
8. |
9. |
|
Solution A |
0 |
1 |
2 |
3 |
4 |
5 |
6 |
7 |
8 |
|
Solution B |
1 |
1 |
1 |
1 |
1 |
1 |
1 |
1 |
1 |
|
Deionised water |
8 |
7 |
6 |
5 |
4 |
3 |
2 |
1 |
0 |
|
T (%) |
|
|
|
|
|
|
|
|
|
|
A |
|
|
|
|
|
|
|
|
|
|
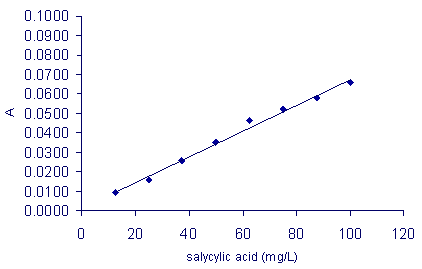
Concentration of salicylic acid (mg/L) |
- |
12.5 |
25.0 |
37.5 |
50.0 |
62.5 |
75.0 |
87.5 |
100 |
- For measuring the transmittance use green LED.
Write the measurements in the table above and calculate the absorbance.
Prepare a calibration curve:
Calibration curve
Hydrolysis of Aspirin tablet
- Weigh 1 tablet of Aspirin 500 and dissolve it in 10 mL of mixture ethanol/water 1:1.
- Filter the solution into a 250 mL volumetric flask, rinse the filter paper with 10 mL solution ethanol/water 1:1 and dilute the solution to 250 mL with deionised water. Transfer this solution into a small plastic reagent bottle.
- Prepare
a blank (1
 of solution B and
8
of solution B and
8 of deionised water) and set
the transmittance at the green
LED to 100%.
of deionised water) and set
the transmittance at the green
LED to 100%. - Put
into the next hole of the blister
8
 droplets of Aspirin
solution and add 1
droplets of Aspirin
solution and add 1 droplet
of solution B.
droplet
of solution B. - Record the transmittance of the Aspirin sample. This is the transmittance of Aspirin solution obtained immediately after the solution preparation.
- Repeat the measurement of the transmittance of Aspirin solution, e.g. after 3 hours, 24 hours and 48 hours. Before the measurement, set the transmittance to 100 % using the blank as described in item 3.
Calculate the absorbance from the transmittance and write the results in the table below.
| Sample of aspirin solution |
Transmittance
|
Absorbance |
| Taken immediately after the preparation |
98.8 |
0.005 |
| Taken after 3 hours |
98.0 |
0.009 |
| Taken after 24 hours |
94.0 |
0.025 |
- Using the calibration curve, discuss what the reason for the increase in absorbance is.
- Repeat the experiment of Aspirin hydrolysis, but this time keep the small reagent bottle with the Aspirin solution in the water bath at elevated temperature (e.g. 70 °C) and record the temperature of the water bath and the time.
- Compare the results obtained at ambient temperature and at elevated temperature, and discuss the differences.
Worked example
Ambient temperature
Absorbances of the Aspirin solution at ambient temperature were: 0.005 after dissolving, 0.009 after 3 hours, and 0.025 after 24 h. These results show that at ambient temperature acetylsalicylic acid, the active ingredient of Aspirin, undergoes hydrolysis, very slowly, and the concentration of salicylic acid, which forms the coloured complex with the reagent is increasing with time.
Elevated temperature
Absorbance after heating the Aspirin sample solution for 15 minutes in a water bath at 70 °C was: 0.016, after heating for 30 minutes it was 0.032, after 45 minutes 0.071, and after 60 minutes 0.090. These results show that the rate of hydrolysis is temperature dependant; at elevated temperature the hydrolysis of acetylsalicylic acid is much quicker than at ambient temperature.
Developed
and prepared
by: Margareta Vrtačnik*,
Vida Mesec* and Nataša Gros**
*University
of Ljubljana, Faculty of Natural
Sciences and Engineering
**University
of Ljubljana, Faculty of Chemistry
and Chemical Technology