|

1. PERISTALTIC PUMP TYPE
This contribution describes
the up-grading of the spectrometer
SpektraTM into simplified
liquid chromatograph for educational
purposes. The chromatograph
was tested for the separation
of dyes in natural Mint aroma.
Apparatus and equipment
- spectrometer SpektraTM,
- a peristaltic pump with
tubes for 0.25 ml/min flow
rate. We used ISMATEC MS-CA2840
pump with constant
number of turns, a Tygon
tubing with internal
diameter of Φi 0.25
mm.
- a transparent polymer
tubing with 0.8 -1.0 mm
internal diameter for making
a flow cell for the spectrometer
and for joining the parts
of the chromatograph,
- a 2 ml Pasteur pipette
PLASTIBRAND with the top
of the bulb cut off and
a tip cut off so that only
approx. 0.5 cm of
narrowed tubing is left
- a laboratory stand,
clamps,
- 50 ml measuring flask,
- 100 ml volumetric flask,
- 25 ml beaker,
- a larger syringe,
- a tubing to connect
the tip of the syringe
with the lower end of the
column,
- a micropipette for dispensing
20 ml
volumes, or, in a simplified
version a dropping bottle
which allows for dispensing
drops of comparable volumes,
- a dropper,
- a stopwatch,
- (optional: a computer
with interface for automatic
data acquisition, or an
integrator).
Reagents
- ammonia (25 %),
- butane-1-ol (ρ
= 0.808 kg/l),
- propane-1-ol (ρ = 0.8 kg/l),
- Silica-gel (Fluka, Kieselgel
60, 230-400 mesh, particle
size from 0.04 mm to 0.063
mm),
- sand,
- glass wool,
- Mint, natural aroma
(Tovarna arom in eteričnih
olj, d.d.).
Hazards
|
|
Ammonia
causes burns. Dangerous
for aquatic organisms.
In contact with
skin or eyes irrigate
with plenty of water
and seek medical
help. Wear protective
glasses and gloves.
Do not
dispose of the liquid
into the environment.
Risk phrases:
34-50, Safety phrases:
26-36/37/39-45-61
|
|

|
|

|
Butane-1-ol
is a flammable and
harmful substance
if ingested. It
is a skin and respiratory
system irritant
and harmful in contact
with eyes. The fumes
can cause dizziness
and confusion. Keep
the chemical in
air-tight containers
and well ventilated
places. If in contact
with eyes, irrigate
with plenty of water
and seek medical
help. If ingested,
seek medical help
and show safety
signs on the label.
Wear protective
glasses and gloves.
Do not dispose of
the liquid into
the environment.
Risk phrases:
10-22-37/38-41-67,
Safety phrases:
7/9-13-26-37/39-46
|
 |
Propane-1-ol
is flammable and
irritant. If inhaled
it causes ataxia,
confusion, nausea,
headache, inebriation.
It causes dryness
of skin and skin
redness and blurred
vision. Use protective
glasses and gloves.
Risk phrases:
11-41-67, Safety
phrases: (2-)7-16-24-26-39
|
|

|
Preparing the mobile phase
Prepare the mobile phase
in a 100 ml flask according
to the volumes given in
the table below.
Table 1.
Volumes for preparing the mobile
phase
| deionized
water
|
propane-1-ol
|
ammonia
(25 %)
|
butane-1-ol
|
| 15 ml
|
45
ml
|
7.5 ml
|
25
ml
|
Stir the solution well and
keep it in a sealed flask.
Assembling the liquid
chromatograph
Use a transparent polymer
tubing as a flow cell, which
will be inserted into the measuring
site in the SpektraTM
spectrometer. The diagram for
making a flow cell is shown
in Picture 1. Insert the flow
cell into the measuring site
and fix it, making sure that
it stays in place during the
experiment.
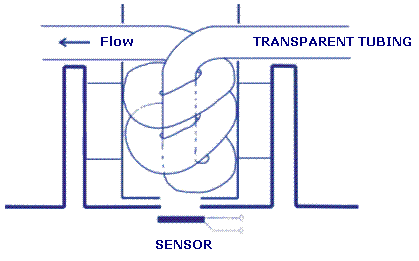
Picture
1. Scheme of the flow cell
Use the Pasteur pipette (it
is described in the »Apparatus«
section above) as a column.
Insert a plug made of glass
wool into the lower part of
the column (do not touch glass
wool by hands, use tweezers!).
Mix in a beaker 5 g of Silica-gel
and 10 ml of the mobile phase.
Fill the column with the prepared
mixture of Silica-gel and mobile
phase. Compact the Silica-gel
layer with the help of the syringe
which is connected with the
bottom part of the column causing
underpressure. Make sure that
the level of the mobile phase
does not fall below the level
of Silica-gel. The Silica-gel
in the column prepared according
to the described procedure,
should not be more than 2.5
cm high. Cover the Silica-gel
layer with a 0.5 cm layer of
finely ground sand. Fix the
column on the stand and attach
it to the inlet tubing of the
flow cell. Connect the outlet
of the flow cell to the peristaltic
pump tube. Place the other end
of the tube of the peristaltic
pump into the beaker which serves
as a receiver for discard solution.
Protect the measuring chamber
with appropriate cover (i.e.
aluminium foil) in order to
prevent the effect of the surrounding
light on the measuring values
of the spectrometer. See the
picture of the assembled chromatograph
on Picture 2. Optionally you
can connect the analogue output
of the spectrometer via a suitable
interface with a computer, or
use an integrator. Measurements
can be taken manually as well.

Picture
2. Liquid chromatograph
Using the chromatograph
for separating the dyes of natural
Mint aroma
Natural Mint aroma contains
E102 and E131 dyes. Search
the Internet to find the information
about these dyes. After
you have assembled the chromatograph
and checked that the flow of
the mobile phase is 0.25 ml/min,
you can start with separation
of the dyes.
The column must be filled
with the mobile phase. Switch
on the spectrometer and select
blue or red LED. Set the intensity
of the light emitter to its
maximum. Switch on the
pump and propel through the
system the mobile phase until
its volume equals to the triple
volume of the stationary phase.
Set the transmittance
to 100.0. When the level of
the mobile phase has reached
the level of the sand dispense
20 ml of natural Mint aroma
onto the top of the sand layer
and start the stopwatch. Use
a dropper, carefully adding
the mobile phase along the wall
of the column, taking care that
the level of the liquid does
not fall under the level of
Silica-gel. Record the results
in 10 seconds intervals.
The separation of components
in the column is shown in Picture
3.
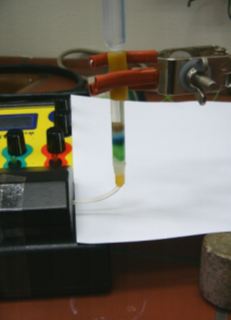
Picture
3. Separating dyes in the
column
Convert the transmittance
into absorbance and draw a graph.
Since the components are not
equally coloured you need to
repeat the experiment, using
another LED (if you used blue
LED first, take red LED next).
Picture 4 shows two
examples of chromatograms obtained
by computerized data acquisition
and measurement.
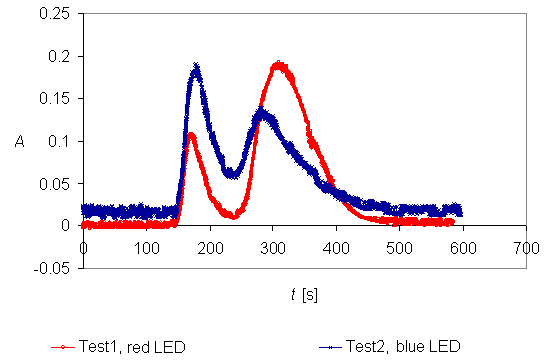
Picture
4. Chromatograms which were
obtained by using blue
and red LED.
Developed and prepared
by: Nataša Gros and Domen
Klančar, University of Ljubljana,
Faculty of Chemistry and Chemical
technology
|