Put the filled blister on a shaker to prevent cell gathering on the bottom of the hole. Set the shaker on 320 RPM (rotations per minute). Blister should be left on the shaker, until the spectrometer is ready to use. Get the spectrometer ready and turn on the blue LED light at the highest intensity. Put the filled blister on spectrometer. Set the transmittance measured at the sterilized culture media on 100. 0 %. After that measure the transmittance of other samples. If measuring lasts too long, the blister should be set on the shaker again, because the cells will begin to gather on the bottom of the blister hole. This affects measuring results. Write all the measured and calculated results in the given table.
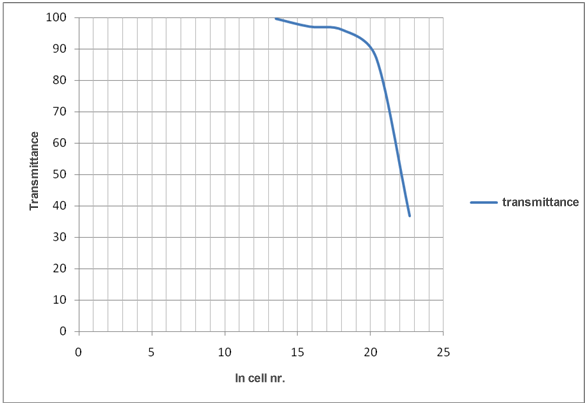
These results are used to create the graph of the calibration curve which shows how changing ln cell number affects transmittance. Measurements used to determine the cell number
|
sample/dilution |
cell nr. /mL |
ln cell nr./mL |
T |
A |
initial |
20.24 . 109 |
23.7 |
42.7 |
0.3696 |
10-1 |
20.24 . 108 |
21.4 |
84.6 |
0.0726 |
10-2 |
20.24 . 107 |
19.1 |
95.6 |
0.0195 |
10-3 |
20.24 . 106 |
16.8 |
95.3 |
0.0209 |
10-4 |
20.24 . 105 |
14.5 |
98.3 |
0.0075 |
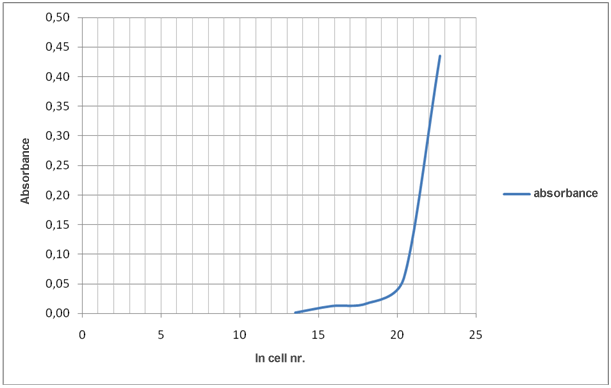
Evaluation of the results in table 1 shows that decreasing number of cells increase transmittance and decrease absorbance.
Transmittance is related to absorbance as described by the equation:
A = –log T
Graph 1: How ln cell nr./mL affects transmittance
Graph 2: How ln cell nr./mL affects absorbance
Example 2:
Table 2: How the number of yeast cells in samples affects transmittance measured in the same samples
sample/dilution |
cell nr./mL |
ln cell nr./mL |
T |
A |
initial |
7.23 . 109 |
22.7 |
36.7 |
0.4353 |
10-1 |
7.23 . 108 |
20.4 |
87.1 |
0.0599 |
10-2 |
7.23 . 107 |
18.1 |
96.0 |
0.0177 |
10-3 |
7.23 . 106 |
15.8 |
97.1 |
0.0128 |
10-4 |
7.23 . 105 |
13.5 |
99.6 |
0.0017 |
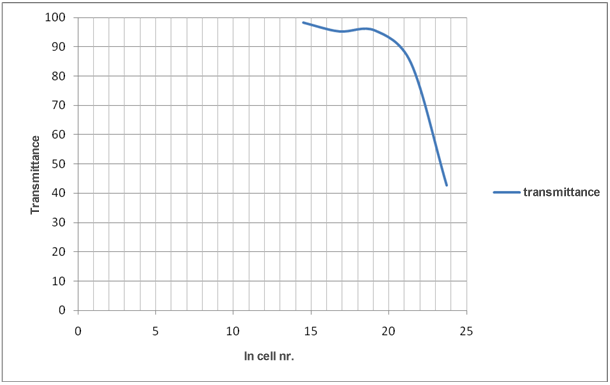
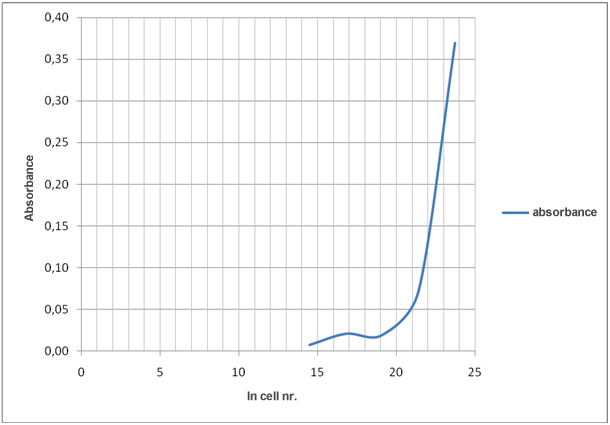
Evaluation of the results in the table 1 shows that decreasing number of cells increase transmittance and decrease absorbance.
Transmittance is related to absorbance as described by the equation:
A = –log T
Graph 3: How ln cell nr./mL affects transmittance
Graph 4: How ln cell nr./mL affects absorbance
Prepared by: Alma Kapun Dolinar