|
VK2Cr2O7 mL |
VH2SO4 mL |
VH2O |
Vethanol mL |
Vdistillate of wine |
vol% |
T % |
T |
A |
|
Calibr.sol.1 |
180 |
110 |
100 |
0 |
0 |
0 |
|
|
|
Calibr.sol.2 |
180 |
110 |
80 |
20 |
0 |
3,84 |
|
|
|
Calibr.sol.3 |
180 |
110 |
60 |
40 |
0 |
7,68 |
|
|
|
Calibr.sol.4 |
180 |
110 |
40 |
60 |
0 |
11,52 |
|
|
|
Calibr.sol.5 |
180 |
110 |
20 |
80 |
0 |
15,36 |
|
|
|
Calibr.sol.6 |
180 |
110 |
0 |
100 |
0 |
19,20 |
|
|
|
Sample |
180 |
110 |
0 |
0 |
100 |
|
|
|
|
RESULTS OF THE EXPERIMENT
The calibration line is a function of the absorbance versus the volumetric part of ethanol (%) in wine. The volumetric part of ethanol in the sample can then be deduced.
Table 2: The results
VK2Cr2O7 mL |
VH2SO4 mL |
VH2O |
Vethanol mL |
Vdistillate of wine |
vol% |
T % |
T |
A |
|
Calibr.sol.1 |
180 |
110 |
100 |
0 |
0 |
0 |
100 |
1 |
0 |
Calibr.sol.2 |
180 |
110 |
80 |
20 |
0 |
3,84 |
99,0 |
0,99 |
0,004365 |
Calibr.sol.3 |
180 |
110 |
60 |
40 |
0 |
7,68 |
96,9 |
0,969 |
0,013676 |
Calibr.sol.4 |
180 |
110 |
40 |
60 |
0 |
11,52 |
94,7 |
0,947 |
0,02365 |
Calibr.sol.5 |
180 |
110 |
20 |
80 |
0 |
15,36 |
90,3 |
0,903 |
0,044312 |
Calibr.sol.6 |
180 |
110 |
0 |
100 |
0 |
19,20 |
88,2 |
0,882 |
0,054531 |
Sample |
180 |
110 |
0 |
0 |
100 |
|
94,1 |
0,941 |
0,02641 |
Chart 1: The absorbance versus the volumetric part of ethanol (%).
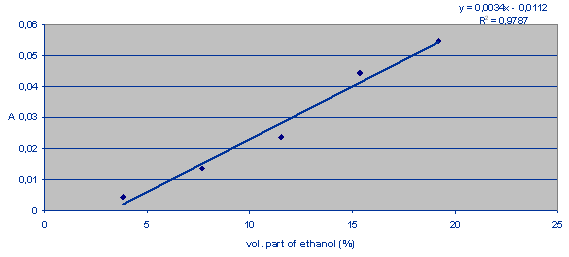
CALCULATION
Asample = 0,02641
Volumetric part of ethanol (%) = 11,06......... from the calibration line
y = 0,0034x-0,0112
x = (y+0,0112)/0,0034 = (0,02641+0,0112)/0,0034
x = 11,06%
In our wine sample was 11,06 volumetric part of ethanol (%).
Developed and prepared by: mag. Marjana Dvoršek, Tadeja Polajnar, Kristina Frlic, Biotechnical Centre Naklo






