
13.
Determining low levels of ethanol in fruit juices
BACKGROUND
Fruit and vegetable products contain low levels of ethanol. The international standard ISO 2448 defines a procedure for determining ethanol in such samples and comprises a distillation and a titration step. The distillation step is essential for eliminating the matrix effect but can also act as a pre-concentration step. The ethanol in the distillate is oxidised with the potassium dichromate(VI) in the presence of the sulfuric(VI) acid. The amount of the remaining dichromate is determined with a titration with the ammonium-iron(II) sulfate(VI) standard solution in the presence of the iron(II)-1,10 phenanthroline as an indicator. In this activity the titration step is replaced with the spectrometric measurement after the reaction of the ethanol with the potassium dichromate.
TASK
Using the SpektraTM spectrometer determine the concentration of ethanol in a fruit juice distillate after reaction with the standard potassium dichromate solution in the presence of sulphuric(VI) acid and calculate the amount of ethanol in a kilogram of the fruit juice.
CONSUMABLES AND EQUIPMENT
For the activity the following items are required:
- analytical balance,
- beaker 800 mL,
- pH meter with combined pH electrode,
- distillation flask 2000 mL,
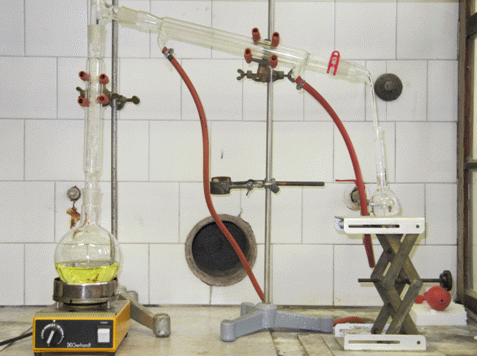
- distillation apparatus (Figure 1),
- volumetric flask 100 mL,
- measuring cylinder 10 ml,
- thermometer,
- boiling chips,
- volumetric flask 1000 mL,
- beakers of different size,
- glass rod,
- quantitative funnel,
- measuring cylinder 500 mL,
- micropipette (50-1000 µL),
- micropipette (100-5000 µL),
- SpektraTM spectrometer,
- glass test-tubes with caps,
- blisters,
- calcium oxide, CaO, (M = 56.08 g/mol),
- potassium dichromate, K2Cr2O7, (M = 294.19 g/mol),
- sulfuric(VI) acid, H2SO4, (M = 98.08 g/mol, ρ = 1.84 g/ml, w = 0,96),
- aluminium powder, Al, (M = 26.98 g/mol),
- ammonium-iron(II) sulfate hexahydrate, (NH4)2Fe(SO4)2·6H2O, (M = 392.14 g/mol),
- iron(II) sulfate heptahydrate, FeSO4·7H2O, (M = 278.02 g/mol),
- 1,10- phenanthroline monohydrate, C12H8N2·H2O,(M = 198,2 g/mol).
HAZARDS

|
Calcium oxide
R: 41
Risk of serious damage to eyes.
S: 22-24-26-39
Do not breathe dust. Avoid contact with skin. In case of contact with eyes, rinse immediately with plenty of water and seek medical advice. Wear eye/face protection. |



|
Potassium dichromate
R:45-46-60-61- 8-21-25-26-34-42/43-48/23-50/53
May cause cancer. May cause heritable genetic damage. May impair fertility. May cause harm to the unborn child. Contact with combustible material may cause fire. Also harmful in contact with skin. Also toxic if swallowed. Also very toxic by inhalation. Causes burns. May cause sensitization by inhalation and skin contact. Also toxic: danger of serious damage to health by prolonged exposure through inhalation. Very toxic to aquatic organisms, may cause long-term adverse effects in the aquatic environment.
S: 53-45-60-61
Avoid exposure - obtain special instructions before use. In case of accident or if you feel unwell, seek medical advice immediately (show the label where possible). This material and its container must be disposed of as hazardous waste. Avoid release to the environment. Refer to special instructions/ Safety data sheets. |
|
Sulphuric acid
R: 35
Causes severe burns.
S: 26-30-45
In case of contact with eyes rinse immediately with plenty of water and seek medical advice. Never add water to this product. In case of accident or if you feel unwell, seek medical advice immediately (show the label where possible).
|
 |
Ethanol
R: 11
Highly flammable.
S: 7-16
Keep container tightly closed. Keep away from sources of ignition - No smoking.
|
 |
Iron(II) sulfate heptahydrate
R: 22-36/38
Harmful if swallowed. Irritating to eyes and skin. |
 |
Aluminium powder
R 15/11
Highly flammable. Contact with water liberates extremely flammable gases.
S 43
In case of fire, use sand. Never use water.
|
PROCEDURE
Preparation of the calcium hydroxide suspension 0.1964 mol/L
In a 500 mL beaker weigh 110 g of calcium oxide and add deionised water. Transfer the suspension quantitatively into a 1000 mL volumetric flask and dilute the suspension with deionised water to the mark.
Preparation of the stock standard solution of potassium dichromate 0.1447 mol/L
Weigh in a beaker 42.5723 g of the potassium dichromate previously dried for two hours at 105 °C. Add deionised water and dissolve the potassium dichromate in the ultrasonic bath. Transfer the solution quantitatively into 1000 mL volumetric flask and dilute the solution with deionised water to the mark.
Preparation of the sulfuric(VI) acid (1+1)
With a measuring cylinder measure 200 mL of deionised water into a 1000 mL beaker. Put the beaker into a water bath and slowly and cautiously add into the beaker 200 mL of concentrated sulfuric(VI) acid.
Distillation
Weigh 700 g of a fruit juice into an 800 mL beaker and measure the pH. Regulate the pH value on 8.0±0.2 by adding the calcium hydroxide suspension. Transfer the solution quantitatively into 2 L distillation flask, add boiling chips and assemble the distillation apparatus as demonstrated in Figure 1. Use a 100 ml volumetric flask filled with 10 mL of deionised water for collecting the distillate, keep temperature between 15 °C and 20 °C to prevent the evaporation of the ethanol. After collecting approximately 80 mL of the distillate stop the distillation. Disassemble the distillation apparatus and wash of the remains of the distillate from the internal walls with few ml of deionised water into the volumetric flask. Dilute the distillate in the volumetric flask to the mark with deionised water and cap the flask.
Determining the ethanol by measuring the absorbance of the potassium dichromate solution after the reaction with the solution of ethanol
With a micropipette measure 0.3 mL of the standard potassium dichromate solution 0.1447 mol/L into a test-tube, add 2 mL of the sulfuric(VI) acid solution (1+1) and 1 mL of the ethanol calibration solution or a distillate of the fruit juice. Prepare the blank similarly by replacing the volume of the ethanol solution and the standard potassium dichromate solution with deionised water. Stopper the test-tubes. The test tubes with the potassium dichromate in the sulfuric acid solution before and after adding the ethanol solution are presented in Figures 2 and 3 respectively. The ethanol was allowed to react with the dichromate for 30 minutes.

Figure 2: The test-tubes with 0.3 mL of the potassium dichromate solution and 2 mL of the solution of the H2SO4 (1+1) before the ethanol solution addition.
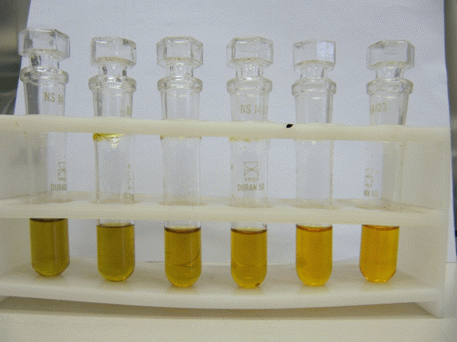
Figure 3: The test-tubes with 0.3 mL of the potassium dichromate solution and 2 mL of the solution of the H2SO4 (1+1) 30 minutes after the addition of 1 mL of the ethanol solutions with different concentrations.
30 minutes after adding the ethanol solution into a test-tube transfer 400 µL of the solutions into the hollows of a blister and measure the transmittance with the SpektraTM spectrometer against the blank using the blue LED. The measurements are presented in Table 1.
Table 1: Transmittance and absorbance of the solutions of dichromate measured against the blank using the blue LED 30 minutes after the addition of the calibration solutions with different ethanol concentrations or after the addition of the distillates of the samples
c (C2H5OH) (mmol/l) |
0 |
3.4 |
10.5 |
17.5 |
24.5 |
31.5 |
38.5 |
45.5 |
black-berry
juice |
pine-apple juice |
T |
20.9 |
21.3 |
22.1 |
25 |
27.2 |
31.3 |
34.5 |
41.4 |
23.3 |
20.0 |
A |
0.6799 |
0.6716 |
0.6556 |
0.6021 |
0.5654 |
0.5045 |
0.4622 |
0.3830 |
0.6326 |
0.6990 |
RESULTS
The dichromate absorbance dependence on the ethanol concentration is presented in Figure 4. The ethanol concentration in distillates can be read from the graph using the absorbance measurements or calculated from the equation. The results calculated from the data in Table 1 are summarised in Table 2.
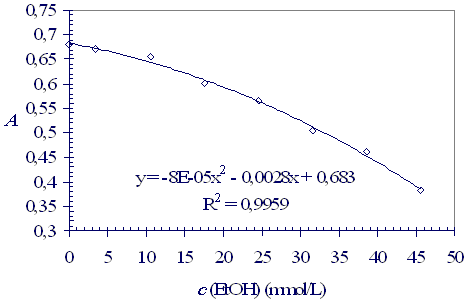
Figure 4: The dichromate absorbance dependence on ethanol concentration
Table 2: The ethanol concentration in the fruit juice distillates
Distillate |
c (EtOH) (mmol/L) |
Blackberry juice |
13.1 |
Pineapple juice |
0 |
The amount of ethanol per kilogram of the fruit juice is calculated from the concentration of ethanol determined in the distillate (c(EtOH)) by multiplying the concentration with the distillate volume (0.1 L) and dividing by the mass of the fruit juice taken for the distillation (mjuice) as demonstrate in the following equation.
n(EtOH) |
= |
c(EtOH)·0.1L |
|
|
kg |
mjuice |
A kilogram of the blackberry juice contains 1.87 mmol of the ethanol.
Developed and prepared by: Dr. Nataša Gros and Karmen Lampreht, University of Ljubljana, Faculty of Chemistry and Chemical Technology, Slovenia.
|




