|

EFFECT OF STRAY LIGHT ON LAMBERT-BEER'S LAW
Background
A monochromator which enables the selection of the wavelength of light is an important part of professional spectrometers. The monochromator comprises an entrance slit through which the light emitted by a light source enters. An optical prism or a diffraction grating inside the monochromator disperses the light into a spectrum. The wavelength of the light which comes out of the monochromator through the exit slit can be selected by changing the position of the optical prism or the diffraction grating what is achieved by changing the position of the wavelength selection potentiometer or through a computerised regulation. Different optical effects can result in the light of the selected wavelength being emitted along with the light of a very different wavelength, so called "stray light". This is undesirable because it affects the results of the spectrometric measurements.
Task
Using the SpektraTM spectrometer find out how the stray light affects the results of spectrometric measurements and the Lambert-Beer’s law.
Consumables and equipment
For the activity the following items are required:
- Copper sulfate, CuSO4·5H2O,
- volumetric flask 50 ml (3x),
- volumetric flask 20 ml (2x),
- volumetric flask 10 ml,
- piston micropipette,
- blister,
- SpektraTM spectrometer.
Hazards

|
CuSO4· 5H2O
Harmful if swallowed. Irritating to eyes and skin. Very poisonous for water organisms: can cause long lasting effects on water environment. Do not breathe dust.
R: 22-36/38-50/53
S: 22-60-61
|
 |
Procedure
Prepare the solutions of the copper sulfate with different concentrations by weighing CuSO4·5H2O and dissolving the chemical in the volumetric flasks as summarised in Table 1.
Table 1: Preparation of the solutions of the copper sulfate with different concentrations
c (mol/L) |
m(CuSO4·5H2O) (g) |
V(flask) (ml) |
0.100 |
1.2425 |
50 |
0.200 |
2.4906 |
50 |
0.300 |
3.7408 |
50 |
0.400 |
1.9916 |
20 |
0.500 |
2.4192 |
20 |
0.600 |
1.4920 |
10 |
Transfer 400 µl of deionised water into the first hollow of a blister. Proceed with the copper sulfate solutions in the same order as written in table 1. Measure the transmittance of the copper sulfate solutions against water by repeating the whole measuring procedure at three different settings of the light source.
Accomplish the first experiment at the highest light intensity of the red LED, the second at the highest intensity of the green LED and the third at the highest intensity of the red LED and low intensity of green LED. The experimental conditions for all three experiments are summarised in Table 2. Write the results in Table 3. Calculate the absorbance and draw a graph as demonstrated in Figure 1. Consider how the stray light affects the results of the measurements and the Lambert-Beer’s law.
Table 2: Setting the LED intensity for the first, the second and the third experiment
|
Experiment 1 |
Experiment 2 |
Experiment 3 |
Light source |
Red LED (R-LED) |
Green LED (G-LED) |
G-LED and R-LED |
The setting of the potentiometer and the LED intensity |

|
|
|
Table 3: Measurements
c (mol/L) |
0.100 |
0.200 |
0.300 |
0.400 |
0.500 |
0.600 |
T (R-LED) |
|
|
|
|
|
|
T (G-LED) |
|
|
|
|
|
|
T (R-LED & G-LED) |
|
|
|
|
|
|
A (R-LED) |
|
|
|
|
|
|
A (G-LED) |
|
|
|
|
|
|
A (R-LED & G-LED) |
|
|
|
|
|
|
Explanation
The results are presented in Figure 1. The copper sulfate solutions are greenish-blue, there fore they absorb red light (R-LED) very intensively. On the other hand, the absorbance of the green light (G-LED) is low. The presence of the stray - green light in the red light decreases the absorbance measurements. The Lambert-Beer’s law exhibits linear relationship between the absorbance and the concentration. The stray light affects the Lambert-Beer’s law by decreasing the slope of the line and consequently the sensitivity of the spectrometric analytical method.
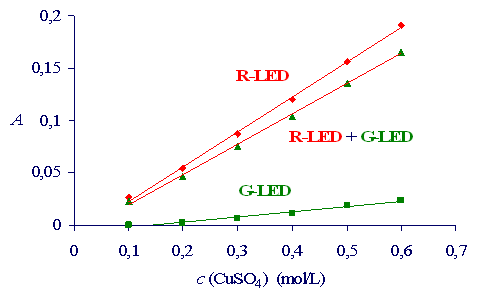
Figure 1: The absorbance in dependence of the concentration of the copper sulfate solutions for experiments performed at different settings of the light source.
Developed and prepared by: Dr. Nataša Gros and Karmen Lampreht, University of Ljubljana, Faculty of Chemistry and Chemical Technology, Slovenia. |