Preparing the mobile phasePrepare the mobile phase in a 100 ml flask according to the volumes given in the table below. Table 1. Volumes for preparing the mobile phase
Stir the solution well and
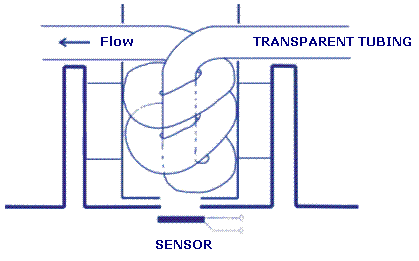
keep it in a sealed flask. Assembling the liquid chromatographUse a transparent polymer tubing to make a flow cell and insert it in the measuring site of spectrometer SpektraTM. The scheme of the flow cell is shown in Picture 1 below. Fix the flow cell so that it says in place.
Picture 1: Flow cell Use the Pasteur pipette (it is described in the »Apparatus« section above) as a column. Insert a plug made of glass wool into the bottom part of the column (do not touch glass wool by hands, use tweezers!). Make a mixture of 5 g Silica-gel and 10 ml of mobile phase in a beaker. Fill the column with the prepared mixture of Silica-gel and mobile phase. Compact the Silica-gel layer with the help of the syringe which is connected with the bottom part of the column causing under pressure. Make sure that the level of the mobile phase does not fall below the level of Silica-gel. The Silica-gel in the column prepared according to the described procedure, should not be more than 2,5 cm high. Cut the pipette transversally, approx. 2 mm above the level of Silica-gel. Reconnect both ends with a rubber tubing allowing 2 mm distance between the two parts of the column. Cover the layer of Silica-gel with 0.8 cm of sand. Attach the column to a stand and connect it with the inlet tube of the flow cell. Attach a separating funnel on the stand just above the column so that you can add the mobile phase into the column. Connect the outlet tube of the flow cell with the vacuum flask through the opening in the rubber tubing. Connect the vacuum flask with the rubber tubing to a 3-way stopcock, and attach a vacuum pump. Protect the measuring chamber with appropriate cover (i.e. aluminium foil) in order to prevent the effect of the surrounding light on the measuring values of the spectrometer. An example of the assembled chromatograph is shown in Picture 2. Optionally you can connect the analogue output of the spectrometer via a suitable interface with a computer, or use an integrator. You can also record measurement values manually. Picture 2. Liquid chromatograph
Using the chromatograph for separating the dyes from Pear aromaPear aroma contains E102 and E110 dyes. After assembling the chromatograph, adjust the flow of the mobile phase to 0.18 ml/min. This can be achieved by inserting the suitable adaptor into the third free way of the stopcock so as to reduce appropriately the under-pressure in the vacuum flask. Picture 3. Injecting a sample into the chromatograph. For this purpose you can use a tip for a micropipette, a conical part of which is transversally cut off so that you get an opening with a desired diameter. Adding the mobile phase from the separating funnel into the column has to be adjusted so that the level of the liquid does not change during the experiment. Switch on the blue LED on the spectrometer. Pump the mobile phase through the system. The volume of the mobile phase for the system, conditioning should be equal to the triple volume of the filling of the column. Set the transmittance to 100.0. Inject 20 µl of the Pear aroma through the rubber tubing into the layer of sand (Picture 3) and start the stopwatch. Record the values in 5 sec intervals and convert the transmittance into absorbance and draw a graph. The chromatogram is shown in Picture 4. Picture 4. A chromatogram
obtained by manual recording
of measurement values taken
in 5-second intervals Developed and prepared by: Nataša Gros and Domen Klančar, University of Ljubljana, Faculty of Chemistry and Chemical technology |