ProcedurePreparation of stock solution: Weigh 0.7191 g of KMnO4, dissolve it in boiled and cooled distilled water and dilute to the mark in a 250 mL volumetric flask. Transfer 10 mL of the reconstituted solution into a 500 mL volumetric flask and dilute contents to the mark. The ready-made solution has a concentration of 20 µg/mL (Mn7+). Decant the prepared solution of manganese ions in to a 10 mL bottle. Preparation of reagents:
Sample preparation: All the measurements should be carried out in triplicate. On an analytical balance weigh about 0.1 g of steel shavings into a 150 mL beaker (accurately record the weight into the chart), add 10 mL of sulphuric acid and some boiling chips. Cover the beaker with the watch glass and heat it on the electric heating plate (in the fume hood) until the steel is dissolved. After dissolution take the beaker off the heating plate and add 2 mL of phosphoric acid. When the solution cools down add 0.5 mL of nitric acid. Don't worry if there are any particles of graphite in the beaker, they do not interfere with the analysis. Heat the solution until the appearance of white steam. Cool the solution down, add about 20 mL of distilled water and two laboratory tables of potassium iodate(VII). Again heat the solution on the electric heating plate. If manganese is present in steel as an alloying element, the solutions turn purple. Quantitatively transfer contents of the beaker into the flask (50 mL) and dilute it to the mark. The resulting solution of the sample is suitable for spectrophotometric analysis. Pour it in a 10 mL bottle. (In the other bottle prepare a few millilitres of prepared solution and add 5 drops of sodium nitrate(III) solution. The solution gets discoloured so you can use it for a blank when calibrating the instrument.) Spectrophotometric measurements:
Table 1:
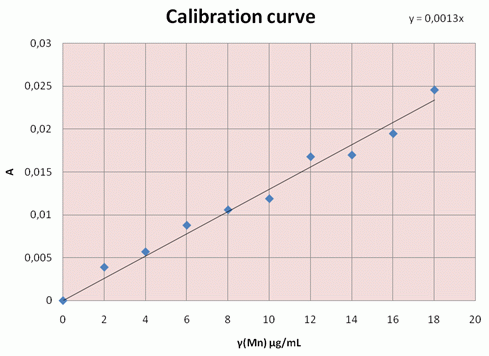
Example of the calibration curve: |
N° of sample |
m of sample [g] |
A |
γ(Mn7+)[µg/mL] |
1 |
|
|
|
2 |
|
|
|
3 |
|
|
|
Calculation
Calculate the percentage by weight of manganese in steel.
w(Mn,%) = |
g (Mn7+).V(sample,aq).100 |
m(sample) |
Result
Table 3:
description of the sample steel |
w(Mn) in steel |
|
|
Developed and prepared by: Romana Mele and Helena Prevc, ŠC SSKŠ Ljubljana