|

CONCURRENT DETERMINATION OF CHROMIUM(III)
AND COBALT(II) IONS
Aim
The aim of this experiment is concurrent determination of chromium(III) and cobalt(II) ions in a sample of water solution using a spectrometric method.
Introduction
1. Background of UV-VIS spectrometry
Electromagnetic radiation passing through a medium may be absorbed, reflected or dispersed. Absorption occurs when the energy of radiation matches the energy gap between two electron energy levels in an absorbing molecule or ion, i.e. when an electron can be transferred from its ground state to the excited state. For electronic transition, the energy of radiation is usually in the range of ultraviolet radiation (UV, 200-380 nm) and visible light (VIS, 380-780 nm). The wavelength of absorbed radiation depends on the electron structure of the molecule or ion. In colourless (white) substances, electron energy levels are rather far apart, so they absorb radiation of higher energy (ultraviolet radiation). In coloured substances, electron energy levels lie closer to one another, therefore such substances absorb visible light.
UV-VIS spectrometry uses absorption of ultraviolet radiation and visible light for analytical purposes. This method is used to study:
- colourless organic compounds that absorb in the UV range (having p or n type electrons, e.g. aromatic hydrocarbons, aldehydes, ketones, carboxylic acids, amines)
- some colourless inorganic compounds (e.g. compounds of rare earth metals, ozone, sulfur dioxide)
- coloured organic compounds (having conjugated systems of double bonds, e.g. organic dyes)
- coloured inorganic compounds (transition metal compounds, such as potassium permanganate or copper(II) sulfate)
- substances that transform to coloured substances as the result of chemical reaction (e.g. formation of complex ions).
2. UV-VIS as a method of quantitative analysis
Intensity of absorption of electromagnetic radiation is characterized by two parameters: transmittance (T) and absorbance (A). Transmittance is defined as the ratio of the intensity of radiation that passed through a sample (I) to the initial intensity of radiation (I0).
T = I/I0
A value of transmittance tells us what portion of radiation has passed trough the sample. Very often, transmittance is given as a percentage: T = I/I0·100%. Absorbance (A) is defined by the following expression:
A = log 1/T = - log T
The Beer-Lambert law states that for a specified wavelength of λ, absorbance Aλ is proportional to the concentration c (in mol dm-3) of the absorbing substance in the solution and to the path length of radiation through the sample l (in cm)
Aλ = ελ·l·c
where ελ is the proportionality coefficient for the wavelength λ, known as the molar extinction coefficient and expressed in dm3 mol-1 cm-1 units. The plot of absorbance against the concentration is linear and the slope of the line corresponds to ελ ·l.
If two or more absorbing species are present in the solution, the Beer-Lambert law predicts that for a given wavelength, values of individual absorbances sum up, as shown below:
A = A1+A2+A3+.....+An = (ε1·c1+ε2·c2+ε3·c3+.....+ε n·cn)·l
Reagents
- Cobalt(II) nitrate hexahydrate, Co(NO3)2 · 6 H2O
- Chromium(III) nitrate nonahydrate, Cr(NO3)3 · 9 H2O
Materials and equipment
- SpektraTM spectrometer
- blisters
- plastic bottles equipped with a dropper
- volumetric flasks 25 cm3 and 50 cm3
Hazards
|
Cobalt(II) nitrate hexahydrate is toxic, may cause cancer and allergy. Avoid contact with skin and inhaling of dust. R: 42/43, 49, 50/53, 60, 68; S: 22, 36/37, 45, 53, 61. |
 |
|
Chromium(III) nitrate nonahydrate is irritating to eyes and skin, it may intensify fire. Avoid contact with eyes and skin. R: 8, 36/38; S: 26. |
 |
Procedure
1. Preparation of standard solutions
Prepare standard solutions separately for cobalt and chromium salts in 50 cm3 volumetric flasks by appropriate dilutions of stock solutions, according to the table below:
Co(NO3)2 · 6 H2O |
65 [g dm-3] |
|
Stock solutions |
Cr(NO3)3· 9 H2O |
120 [g dm-3] |
No. |
Co2+ |
Cr3+ |
V [cm3] |
c [mol dm-3] |
V [cm3] |
c [mol dm-3] |
1. |
0.5 |
|
5 |
|
2. |
1.5 |
|
10 |
|
3. |
2.5 |
|
15 |
|
4. |
3.5 |
|
20 |
|
5. |
4.5 |
|
25 |
|
Write the concentration values in the table.
Hint: Computed concentration values in g dm-3 are transformed to mol dm-3 by division by the molar mass of the corresponding salt (cobalt salt: M1=…………, chromium salt: M2= ……………..).
2. Selection of LED
Measure 10 drops of each solution no. 3 (of cobalt salt and of chromium salt) to the hollows of the blister. Measure transmittance with blue, green and red LEDs. For each salt chose the LED for which the measured value of transmittance is the lowest (i.e. absorbance is the highest!). Use deionized water as a blank.
3. Transmittance measurements for cobalt and chromium salts solutions prepared in part 1
For each cobalt salt solution (five solutions in the table in part 1, 10 drops of each solution) make three measurements of the transmittance using both LEDs selected in part 2 (you should obtain in total 6 measurement results). Repeat the procedure for the chromium salt solution. Put the transmittance values in the table and calculate the values of absorbance (according to the equation A = -log(T/100) or find the values in the table in appendix). Use deionized water as a blank for the measurements:
Transmittance measurements
Solution no. |
T (Co2+ solution) |
T (Cr3+ solution) |
LED 1 |
LED 2 |
LED 1 |
LED 2 |
1 |
|
|
|
|
|
|
|
|
|
|
|
|
2 |
|
|
|
|
|
|
|
|
|
|
|
|
3 |
|
|
|
|
|
|
|
|
|
|
|
|
4 |
|
|
|
|
|
|
|
|
|
|
|
|
5 |
|
|
|
|
|
|
|
|
|
|
|
|
Absorbance values (calculated from transmittance)
Solution no. |
A (Co2+ solution) |
A (Cr3+ solution) |
LED 1 |
LED 2 |
LED 1 |
LED 2 |
1 |
|
|
|
|
|
|
|
|
|
|
|
|
2 |
|
|
|
|
|
|
|
|
|
|
|
|
3 |
|
|
|
|
|
|
|
|
|
|
|
|
4 |
|
|
|
|
|
|
|
|
|
|
|
|
5 |
|
|
|
|
|
|
|
|
|
|
|
|
LED 1 – LED selected for cobalt salt solution – colour .......................
LED 2 – LED selected for chromium salt solution – colour .......................
4. Calibration curves
In the tables below, give the values of concentration of the solutions, then give average values of absorbance each calculated on the basis of three measurements (for LED 1 and LED 2). Use the tables to prepare calibration curves, i.e. plot absorbance A as a function of concentration (A = f(c)). Use Microsoft Excel to generate the graphs.
Table average absorbance values for cobalt salt
Solution
no. |
c [mol dm-3] |
A (average) |
LED 1 |
LED 2 |
1 |
|
|
|
2 |
|
|
|
3 |
|
|
|
4 |
|
|
|
5 |
|
|
|
Table of average absorbance values for chromium salt
Solution
no. |
c [mol dm-3] |
A (average) |
LED 1 |
LED 2 |
1 |
|
|
|
2 |
|
|
|
3 |
|
|
|
4 |
|
|
|
5 |
|
|
|
5. Preparation and absorbance measurements of two-component solutions.
Prepare solutions according to the table below using 25 cm3 volumetric flasks (every solution is a mixture of cobalt and chromium salt). Place 10 drops of every solution in the blister holes and make five measurements for every solution and for both LEDs. Calculate the absorbance values as in part 4 and find the average values for every solution and both LEDs. Give the values in the tables below. Use deionized water as a blank for the measurements.
Stock stolutions |
Solution A |
Solution B |
V [cm3] |
V [cm3] |
Co(NO3)2· 6 H2O 65 [g dm-3] |
0.5 |
4.0 |
Cr(NO3)3 · 9 H2O 120 [g dm-3] |
2.5 |
10 |
H2O |
22 |
11 |
Results of the measurements with the LED 1
Solution
no. |
Solution A |
Solution B |
T |
A |
A (average) |
T |
A |
A (average) |
1 |
|
|
|
|
|
|
2 |
|
|
|
|
3 |
|
|
|
|
4 |
|
|
|
|
5 |
|
|
|
|
Results of the measurements with the LED 2
Solution
no. |
Solution A |
Solution B |
T |
A |
A (average) |
T |
A |
A (average) |
1 |
|
|
|
|
|
|
2 |
|
|
|
|
3 |
|
|
|
|
4 |
|
|
|
|
5 |
|
|
|
|
6. Calculation of concentrations of Co2+ and Cr3+ ions in solutions A and B
Read the slopes of the curve a from the callibration graphs A = a·c
aCo2+, LED1=
aCo2+, LED2=
aCr3+, LED1=
aCr3+, LED2=
For every solution compute concentrations of Co2+ and Cr3+ ions, using the set of equations below (ask your teacher how to derive this set of equations).
ALED1 = aCo2+, LED1 · cCo2+ + aCr3+, LED1 · cCr3+
ALED2= aCo2+, LED2· cCo2+ + aCr3+, LED2 · cCr3+
7. Comparison of the measurement results with expected results
Compute the relative error (Δc) for concentrations determined for Co2+ and Cr3+ solutions (in part 6), using the following equation:
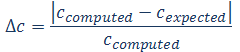
Find the expected concentrations on the basis of composition of solutions A and B (see table in part 5). Computed concentration values in g dm-3 are transformed to mol dm-3 by division by molar mass of corresponding salt (cobalt salt: M1=............, chromium salt: M2=............). Compute the concentrations as in part 1.
8. Concurrent determination of unknown concentration of Co2+ and Cr3+ ions.
Analyze the sample X of unknown concentration of Co2+ and Cr3+ ions according to the procedures described in parts 5 and 6.
Solution
no. |
Sample X (LED 1) |
Sample X (LED 2) |
T |
A |
A (average) |
T |
A |
A (average) |
1 |
|
|
|
|
|
|
2 |
|
|
|
|
3 |
|
|
|
|
4 |
|
|
|
|
5 |
|
|
|
|
Prepared by: Tomasz Chmiel, Tomasz Dymerski, Technikum Przemysłu Spożywczego i Chemicznego w Gdańsku
Revised by: Marek Kwiatkowski, Wydział Chemii Uniwersytetu Gdańskiego
|