|

EVALUATION OF THE EFFICIENCY OF DIFFERENT METHODS FOR THE REMOVAL OF COPPER IONS FROM WASTE-WATERS
During the production of copper from copper ores, and in printed circuit board manufacturing, electronics plating, plating, wire drawing, copper polishing, paint manufacturing, wood preservatives production and printing operations great amounts of waste-waters with high concentrations of copper ions are produced. Copper ions are very toxic to aquatic micro- organisms and, prior to release into the environment, must be removed from the contaminated waters. High concentrations of copper in fresh water streams are also endangering other organisms including humans, since, through the food chain, copper is accumulated into fish and other higher organisms.
Table 1: Copper ores (source: Wikipedia)
Malachite - CuCO3.Cu(OH)2 |
Chalcopyrite
- CuFeS2 |
|
|
Copper ions can be removed from waste-waters by precipitation or reduction. In both processes solid products are formed which, after filtration and pH adjustment, are deposited at industrial waste treatment sites, or recycled.
Your task
School laboratories are also important sources of waste-water contaminated with copper ions, since an aqueous solution of copper(II) sulphate is often used as a reagent in many lab assignments. This water must be appropriately treated before being drained into the lab sinks. Your task is to select the most efficient method for the removal of copper ions from waste-water.
Available reagents for the precipitation and reduction are shown in Scheme 1.
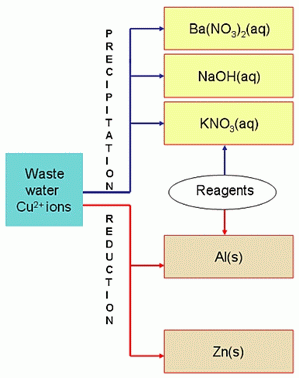
Scheme 1: Reagents for the removal of copper ions
Safety
You must wear a lab coat, protective goggles and gloves.
Ba(NO3)2 – may cause skin and eye irritation; is non-flammable; it does not react with water; yields toxic gaseous oxides of nitrogen when involved in fire.
NaOH – corrosive and toxic upon digestion, skin contact causes burns, longer exposure results in dermatitis; is non-flammable; in water decomposes into sodium and hydroxide ions; water solutions are strong bases
R 35, S 26, 37/39 45
KNO3 – oxidant, skin and eye irritant, non-flammable; it does not react with water; yields toxic gaseous oxides of nitrogen when involved in fire.
Reagents and other laboratory ware:
- Wastewater containing copper ions (provided by the teacher)
- CuSO4 (aq) – 0,5 mol/L
- Ba(NO3)2(aq) – 1 mol/L
- NaOH(aq) – 1 mol/L
- KNO3(aq) – 1 mol/L
- Al foil
- Zinc beads
- Blisters
- Deionised water
- Test tubes and stands
- Plastic reagent bottles with droppers
- Glass rods
- Funnels
- Filter paper
- Spectrometer SpektraTM
Step-wise procedure
1. Preparation of the calibration curve
Use the blister and dispense drops of standard Cu2+ solution and deionised water into the hollows according to the scheme in Table 2.
Table 2: Calibration curve preparation
Solutions |
1. |
2. |
3. |
4. |
5. |
6. |
7. |
8. |
9. |
Blank |
CuSO4(aq) drops
|
1
|
2 |
3 |
4 |
5 |
6
|
7 |
8 |
9 |
|
Deionised water, drops |
8 |
7 |
6 |
5 |
4 |
3 |
2 |
1 |
0 |
9 |
c [mol/L]
|
0.056 |
0.111 |
0.167 |
0.222 |
0.278 |
0.333 |
0.389 |
0.444 |
0.500 |
0.0 |
T
|
|
|
|
|
|
|
|
|
|
|
A
|
|
|
|
|
|
|
|
|
|
|
Measure the transmittance of the solutions against the blank (deionised water), using the red LED and input data into Table 2.
2. Use the Excel computing programme to calculate the absorbance, and generate a graph of the calibration curve; calculate the equation of the best fit line.
Graph 1: Calibration curve
3. Put six test tubes into the stand and add reagents according to the scheme in Table 3.
Table 3: Preparation of the reaction mixtures
Test tube/ reagent
|
1.
Ba(NO3)2(aq) |
2.
NaOH(aq)
|
3.
KNO3(aq)
|
4.
Zn(s)
|
5.
Al(s)
|
6.
Untreated copper ions solution
|
Volume/mass
[ml] or [g] |
4 |
4 |
4 |
2g* |
0.5 g* |
|
Water [ml] |
0 |
0 |
0 |
4 |
4 |
4 |
Cu2+(waste) [ml] |
6 |
6 |
6 |
6 |
6 |
6 |
* Use small beads of Zn to increase the reaction surface area, and aluminium foil, which should be crumpled in order to increase its surface area.
4. Shake the reaction mixtures and wait 15 – 20 minutes in order for clear filtrates to be formed. Filter the content of the 1st and 2nd test-tubes, and if necessary also of the 4th test-tube into plastic reagent flasks with droppers, while clear filtrates are dispensed into reagent flasks.
5. Dispense into the blister's hollows nine droplets of filtrates, and into the last hollow nine droplets of original untreated waste-water. Use deionised water as the blank. Measure the transmittance and calculate the absorbance. Use the calibration curve or equation of the curve for the determination or calculation of the concentration of copper ions in the filtrate after treatment, Table 4.
Table 4: Results after treatment
|
Precipitation |
Reduction |
Untreated |
Filtrate after addition of reagent |
Ba(NO3)2(aq) |
NaOH(aq) |
KNO3(aq) |
Zn(s) |
Al(s) |
Cu2+(waste) |
T |
|
|
|
|
|
|
A |
|
|
|
|
|
|
c [mol/L] |
|
|
|
|
|
|
6. Comment on which method was the most efficient and why:
Checking comprehension
1. Write chemical equations for all reactions in the processes of copper ions removal. Use solubility data in Table 5 and electrochemical potential data (Table 6).
Table 5: Water solubility
Compound |
Solubility |
Ba(NO3)2 |
79 g/L (20°C) |
BaSO4 |
insoluble |
CuSO4.5H2O |
316 g/L (20°C) |
Cu(NO3)2 |
soluble |
NaOH |
1,1 kg/L |
Na2SO4 |
2680 g/L |
Cu(OH)2 |
2,5 mg/L (25°C) |
KNO3 |
357 g/L |
K2SO4 |
11 g/L (20°C) |
Al2(SO4)3 |
870 g/L |
ZnSO4 |
soluble |
Table 6: Electrochemical potential
Metal/cation |
E° (volts) |
Lithium/Li+ |
-3.03 |
Potassium/K+ |
-2.92 |
Calcium/Ca2+ |
-2.87 |
Sodium/Na+ |
-2.71 |
Magnesium/Mg2+ |
-2.37 |
Aluminium/Al3+ |
-1.66 |
Zinc/Zn2+ |
-0.76 |
Iron/Fe2+ |
-0.44 |
Lead/Pb2+ |
-0.13 |
Hydrogen/H+ |
0 |
Copper/Cu2+ |
+0.34 |
Silver/Ag+ |
+0.80 |
Gold/Au3+ |
+1.50 |
2. Which method for the removal of copper ions from waste-water was the most efficient one and why?
3. Comment on the positive and negative aspects of this method.
4. Could the calibration curve which was used for the determination of the concentration of copper ions in the filtrate after treatment, be used for any waste-water containing copper ions regardless of their concentration? Explain.
5. Think about any other method, apart from those already used, which could be also appropriate for treatment of waste-water containing copper ions.
Developed and optimized: Margareta Vrtačnik and Vida Mesec, University of Ljubljana, NTF-KII. |