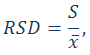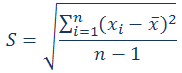|

DETERMINATION OF IRON IN GROUNDWATER
Aim
Determination of iron(III) ions in samples of groundwater using a spectrometric method.
Introduction
In moderately acidic solution, iron(III) ions reacts with thiocyanate (rhodanide) ions SCN- to give red-brown, intensely coloured complex compounds. The reaction is stepwise, so Fe(SCN)2+, Fe(SCN)2+ etc. up to Fe(SCN)63- species form in the solution. The type of the complex prevailing in the solution depends on the concentration of the reagents and the pH. In diluted solutions, usually used in spectrometric analysis, and at comparable initial concentration of iron(III) and thiocyanate ions, the predominant species is the Fe(SCN)2+ ion. An increase of molar ratio SCN- to Fe3+ results in the formation of complexes with a higher number of coordinated ligands. Therefore it is important to use solutions of the same concentration of thiocyanate ions in spectrometric determinations in order to obtain comparable results. Solutions should be moderately acidic (pH above 3) to prevent hydrolysis of iron(III) ions to insoluble iron(III) hydroxide Fe(OH)3.
Quantitative determination of iron in samples of water is based on absorbance measurements of solutions obtained by adding thiocyanate ions. This way the concentration of iron(III) ions can be determined. Total iron content can be also found after oxidizing iron(II) ions in the solution to iron(III). Anions that form stable complexes with iron(III), such as fluoride, phosphate, citrate or oxalate, interfere in this analytical method. Besides, the sample should not contain any metal ions that produce colored thiocyanate complexes, such as cobalt, chromium, molybdenum or titanium ions.
Reagents
- Ammonium thiocyanate NH4SCN
- Nitric acid HNO3
- Ammonium iron(III) sulfate dodecahydrate (NH4)(SO4)2 · 12 H2O
Materials and equipment
- SpektraTM spectrometer
- blisters
- plastic bottles equipped with a dropper
- volumetric flasks 50 cm3
- pipette 25 cm3
Hazards
|
Nitric acid is corrosive. Avoid contact with skin and inhaling vapors. R : 8-35, S: 23-26-36-45. |
|
Ammonium thiocyanate is harmful to respiratory tract, on contact with skin and on ingestion. R: 20/21/22, 32, 52/53; S: 13, 61. |
Procedure
1. Preparation of standard solutions
Prepare the solutions in 50 cm3 volumetric flasks by mixing appropriate amounts of iron(III) salt stock solution with 5 cm3 ammonium thiocyanate solution NH4SCN (20%) and 2 cm3 nitric acid solution HNO3 (2 mol dm-3) and filling the flask with deionized water to the volumetric mark (proportions of the components are given in the table below).
Stock solution of iron(III) salt – water solution of ammonium iron(III) sulfate dodecahydrate Fe(NH4)(SO4)2 · 12 H2O of concentration 0.01 mg Fe cm-3.
VFe [cm3] |
cFe [mg Fe cm-3]
|
VHNO3 (2M) [cm3] |
VNH4SCN (20%) [cm3] |
5 |
0.001 |
2 |
5 |
10 |
0.002 |
15 |
0.003 |
20 |
0.004 |
25 |
0.005 |
2. Selection of LED
Measure 10 drops of the solution containing 0.003 mg Fe cm-3 into the hollow of a blister. Measure the transmittance with blue, green and red LEDs. Chose the LED for which the measured value of transmittance is the lowest (i.e. absorbance is the highest!). Use deionized water as a blank.
3. Transmittance measurements for iron(III) ions solutions prepared in par. 1
For each iron(III) ions solution (five solutions in the table in part 1, 10 drops of each solution) make three measurements of the transmittance using the LED selected in part 2. Put the transmittance values in the table and calculate the values of absorbance (according to the equation A = -log(T/100) or find the values in the table in appendix). Use deionized water as a blank for the measurements.
Results of the measurements
Solution no. |
cFe [mg Fe cm-3] |
T |
A |
A (average) |
1 |
0.001 |
|
|
|
|
|
|
|
2 |
0.002 |
|
|
|
|
|
|
|
3 |
0.003 |
|
|
|
|
|
|
|
4 |
0.004 |
|
|
|
|
|
|
|
5 |
0.005 |
|
|
|
|
|
|
|
4. Calibration curve
Using the data from the table in part 3 prepare the plot of a calibration curve, i.e. plot of absorbance A as a function of concentration (A = f(c)). Use Microsoft Excel to generate the graphs.
5. Determination of iron(III) concentration in a sample of groundwater
Measure out a 25 cm3 sample of ground water into a 50 cm3 volumetric flask, add 5 cm3 of ammonium thiocyanate solution NH4SCN (20%), 2 cm3 nitric acid HNO3 (2 mol dm-3), and fill with deionized water up to the volumetric mark. Place 10 drops of the resulting solution in the hollow of a blister and read transmittance for the LED selected in part 2. Use deionized water as a blank for the measurements. Compute the average value of absorbance.
Measurement no. |
Sample X |
T |
A |
A (average) |
1 |
|
|
|
2 |
|
|
3 |
|
|
4 |
|
|
5 |
|
|
Calculate the concentration of iron(III) ions in the sample of groundwater in the following way:
c = 2 · A/a
a – slope of calibration curve
A – average value of absorbance
c – concentration of iron(III) ions inthe sample of water, expressed in mg Fe cm-3
2 – dilution factor
6. Determination of the reproducibility of the measurements
Calculate the relative standard deviation (RSD) using the following expressions.
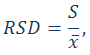 |
where |
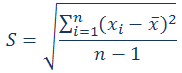 |
RSD – relative standard deviation
S – standard deviation
xi – individual result of the measurement
 – average result of the measurements – average result of the measurements
n – number of results (measurements)
References
- „Chemia Analityczna.Cz. III” J. Minczewski, Z. Marczenko, PWNN, Warszawa 1985.
- „Ocena i Kontrola Jakości Wyników Analitycznych”, CEEAM, Gdańsk 2004.
Prepared by: Tomasz Chmiel, Tomasz Dymerski, Technikum Przemysłu Spożywczego i Chemicznego w Gdańsku
Revised by: Marek Kwiatkowski, University of Gdansk
|