|

UV Spectroscopy
and Sun-Creams
Background Information
Atmospheric diatomic oxygen (O2) blocks out ultraviolet light of approximate wavelengths 220-260 nm and triatomic oxygen (ozone, O3) blocks out approx 240-290 nm.
UV-C is 220-290 nm so stratospheric O2 and O3 block out this very harmful UV-C radiation
UV-B is 290-320 nm. Ozone blocks out some radiation within the range 290-320 nm (absorbed by Ozone but only weakly at these wavelengths) but when the Sun is high with respect to the horizon as in summer, more UV B gets through. With the destruction of the ozone layer, our exposure to UV radiation is increasing.
UV-A is 320-400 nm and there are no major absorbers in the atmosphere in this region. Hence, the need for sun-block for both UV-A and UV-B as these can therefore reach the surface of the Earth and are harmful to our skin. We therefore need to be protected against them. Sun-creams are designed to block out some of this ultraviolet radiation.
How do sun-creams protect our skin from harm?
Sun creams form an artificial barrier that prevents these harmful rays from being absorbed by our skin, thus preventing us from harm. Instead, the sun-cream absorbs or scatters the light energy.
The active component in sun-cream that absorbs UV is excited into a higher energy state, where it can return to its original energy level by the emission of IR radiation. In essence, a sun-cream takes in harmful UV radiation and converts it into harmless heat energy. The compounds that do this are often large organic molecules. Some of the most common are:
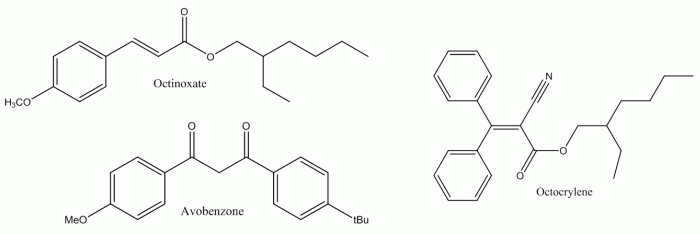
Sun-reams that scatter UV are often inorganic; the most common being titanium dioxide (TiO2) and zinc oxide (ZnO). They scatter light and leave a white film on the skin. These are the types of sun-block favoured by cricketers., This isn’t always the most appealing option. To get round this, manufacturers use very small particle sizes for the oxides and prevent them sticking together by using chemicals to keep them separated.
What makes a good sun-cream?
The Sun Protection Factor (SPF) is a measure of how effective a sun-cream is at preventing damage from sunlight. An SPF 15 cream means that skin can be exposed to fifteen times the amount of UV light before it becomes burnt. Of the UV light that reaches the surface of the Earth, almost 99% is UVA, so it follows that sun creams need to be very effective at absorbing this range of wavelengths. However, UVB is responsible for burning, whereas UVA causes invisible damage such as skin aging and damage to DNA. To be a good sun-cream the product needs to absorb a wide range of UV wavelengths, which is why they contain a complex mixture of chemicals. The more UV light that a cream can absorb or scatter, the better it is at protecting our skin.
Procedure
- Measure 0.1g of your first sun-cream sample into a glass beaker.
- Add 20 ml of ethanol to the beaker and stir well. The sample may need to be heated gently on a hot plate (note no flamea!). It is likely that not all of the sample will dissolve, any white solid left in the beaker is normal.
- Repeat this process for each of the different creams to obtain 5 different solutions.
- Place a clean blister tray into the UV-vis spectrometer and calibrate the intensity so that the display reads 100 once 5 drops of ethanol are added. The devices are very sensitive so will need careful use of the fine tuning control. (See laminated instruction sheet)
- Place five drops of each sample solutions into one of the wells on the sample tray and place in the spectrometer. Record the new value for the transmittance.
- Plot a graph of transmittance vs. Sample to analyse the trends in samples.
Practical considerations
- Any white solid left in the ethanol is a mixture of the insoluble compounds in the cream. It is important to not include any of this in the sample tray as it will affect the results.
- Due to the design of the spectrometers, the path length of the cell is the depth of the solution. Therefore it is important that a similar volume of each solution is added to each well. The results will vary greatly if this is not followed.
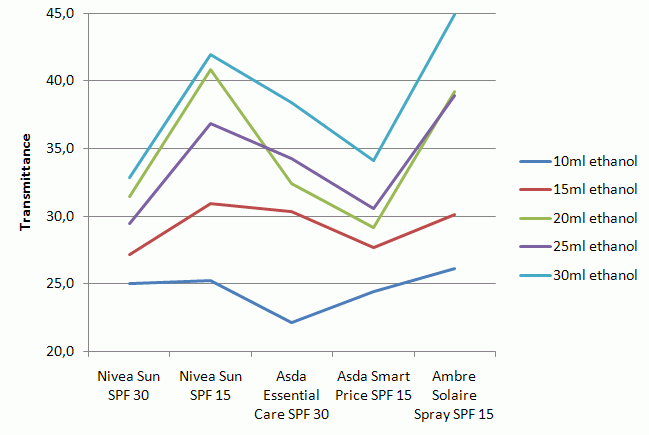
- Because the experiment is not particularly robust, if numerous groups are conducting the experiment at the same time, it may be advisable to carry out the experiment with different volumes of ethanol (i.e. different concentrations). This will make it easier to see the trends in a graph showing all the different concentrations. (see example data)????
Safety Notes
Ethanol
- Highly flammable – keep away from flames and sources of ignition.
- Irritating to skin and eyes – lab coats and goggles to be worn throughout.
- Under no circumstances is laboratory ethanol to be consumed.
UV Spectrometer
- Do not look directly at the UV light source. Only switch on the UV source when the lid is closed onto the sample.
Conclusions
- Not all sun-creams are equal in their ability to absorb ultraviolet light. Just because they claim the same SPF, does not mean they all absorb as effectively or equally.
- The difference between SPF 30 and 15 creams here is not as great as one may expect it to be. Whilst this isn’t a proof, it does suggest that intelligent use of a SPF 15 cream can be just as effective as a higher factor cream.
- SPF is not a 100% precise, scientific way of measuring sun cream strength! This is because it relies on testing on volunteer’s skin, results can vary. An investigation by the consumer magazine ‘Which?’ found that results for SPF could vary by as much as ±6.
Example Answers and Comments
- Calculate the average transmittance for each sun-cream.
- A simple mean is all that is necessary here. See Example Data.
- Plot a graph of your results. On the x-axis, plot the five different creams. On the Y-axis, plot the transmittance, using your average results you calculated in part 1.
- Using your graph, answer the following questions:
- Which sun-cream do you think is the best?
- For this experiment, recognising that the sample with the lowest transmittance would be the best is all that is required. In the case of the example data it is either the Nivea Sun SPF 30 or the Asda Smart Price SPF 15 (a supermarket own brand). The least effective tested by this method is the Ambre Solaire Spray
- The Nivea creams cost £10 each, Ambre Solaire £7 and the Asda creams £3. Which is the best value for money per tube?
- According to the example data; the Asda Smart Price SPF 15.
- What are the limitations and problems of using this technique?
- We can only measure the absorbance caused by compounds that are soluble in ethanol. The samples will most likely contain some white residue, which is likely to contain TiO 2 and ZnO. The latter is one of the most effective at protecting from UVA.
- For the previous reason, the test is biased towards sun-creams that use a majority of organic compounds. It is likely the Ambre Solaire appears so poor partly because it has a higher proportion of inorganic particles.
- By measuring the sample by mass we are not accounting for the variation in concentrations. The most likely reason for the Ambre Solaire’s poor performance is that it is a spray, and would have a greater proportion of solvent to aid its propulsion. A rigourous test would take this into account. Also worthy of note is that this implies a greater application of the spray would be needed, thus reducing its value for money.
- The results are not particularly reliable by nature. Whilst the trends shown below should be observed, actual values can vary strongly due to different path lengths. If a student accidently over filled one of the wells it is likely that this sample would appear the most effective.
- The test was repeated using water as the solvent instead of ethanol. The Ambre Solaire was shown to have a transmittance of approx. 25, whereas the other four samples all had values of approx. 60.
- Why do you think there is such a difference between the values for Ambre Solaire in water and in ethanol?
- This is most likely due to the relative solubilities of the ingredients in the different solvents. Creams that dissolve better in ethanol have a higher concentration of active ingredients and therefore be better at absorbing the UV light, likewise for water. The Ambre Solaire Spray is more soluble in water, and probably uses less of the large organic molecules
- Why could water solubility be a big problem for this product?
- The biggest market for sun-creams is amongst holiday goers and tourists to hot countries. Many users of sun-cream are likely to be on a beach or by a swimming pool, where a water soluble cream would wash off very quickly. A water resistant cream is therefore preferable as it would require fewer applications. A cynical student might remark that this is a bonus for the manufacturer, as it would mean replacing the product more often.!
- Why do you think the other sun-creams show similar transmittance?
- The other four sun-creams probably all contain a similar amount of ingredients that are soluble in water.
Example data
|
|
Transmittance |
Volume of ethanol/ml |
Run |
Nivea Sun SPF 30 |
Nivea Sun SPF 15 |
Asda Essential Care SPF 30 |
Asda Smart Price SPF 15 |
Ambre Solaire Spray SPF 15 |
10 |
1 |
25.8 |
25.2 |
22.2 |
24.4 |
26.2 |
|
2 |
24.8 |
25.4 |
22.2 |
24.5 |
26.2 |
|
3 |
24.5 |
25.2 |
22.1 |
24.3 |
26 |
|
Mean |
25.0 |
25.3 |
22.2 |
24.4 |
26.1 |
15 |
1 |
27.2 |
30.9 |
30.3 |
27.6 |
29.9 |
|
2 |
27.2 |
31 |
30.5 |
27.7 |
30.1 |
|
3 |
27.2 |
31 |
30.3 |
27.7 |
30.3 |
|
Mean |
27.2 |
31.0 |
30.4 |
27.7 |
30.1 |
20 |
1 |
30.9 |
42 |
31.8 |
29.2 |
38.6 |
|
2 |
31.3 |
42.9 |
31.9 |
29.2 |
38.6 |
|
3 |
31.1 |
42.8 |
31.8 |
29.1 |
38.5 |
|
4 |
32.4 |
35.5 |
34.1 |
29.2 |
41.1 |
|
Mean |
31.4 |
40.8 |
32.4 |
29.2 |
39.2 |
25 |
1 |
28.5 |
36.9 |
34.6 |
30.1 |
38.8 |
|
2 |
28.5 |
36.7 |
34.5 |
30.4 |
38.8 |
|
3 |
31.3 |
36.9 |
33.6 |
31.1 |
39.2 |
|
Mean |
29.4 |
36.8 |
34.2 |
30.5 |
38.9 |
30 |
1 |
32.7 |
41.8 |
38.5 |
34.3 |
44.2 |
|
2 |
33 |
42.1 |
38.4 |
33.9 |
45.6 |
|
3 |
32.9 |
42 |
38.4 |
34.1 |
44.9 |
|
Mean |
32.9 |
42.0 |
38.4 |
34.1 |
44.9 |
Student worksheet
Authors: Daniel Wolstenholme and Tim Harrison (Bristol ChemLabS)
|

