|

Determination of vitamin E in a sunflower oil by HPLC
The aim
Namen eksperimenta je določiti vitamin E v sončničnem olju, z uporabo tekočinske kromatografije visoke ločljivosti HPLC.
Introduction
The term Vitamin covers a large group of low molecular organic compounds with various chemical structures, widely distributed in the world of plants and animals. These compounds are necessary factors for growth and support of living functions. For many organisms, including humans, they are usually of exogenous character and must be delivered together with food. The negative health symptoms caused by a deficit of vitamins are called avitaminosis. The state between avitaminosis and a normal level of vitamins in the organism is often called hypovitaminosis. It is usually caused by inappropriate diet, limited assimilation of vitamins from alimentary track or its bacterial infections. Overuse of vitamins, mainly those well soluble in fats may lead to many harmful symptoms generally called hypervitaminosis.
The main classification of vitamins divides them into:
- Fat soluble vitamins
- Water soluble vitamins
Vitamin E
Vitamins that belong to the E group contain and hydroxyphenolic aromatic moiety bound to the tetraisopentenyl chain. Their diversity is due to a different positions of –CH3 groups in the structure. They are resent in large quantities in wheat germs (Triticum sp.), lettuce (Lactuca sativa), soybean (Glycine max). Today, 8 compounds belonging to the two general groups of vitamin E were isolated. These two groups are tocopherols and tocotrienols.
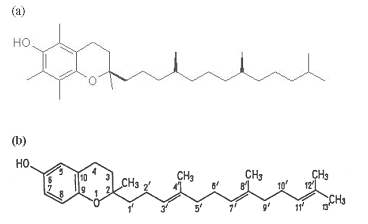
Chemical structures of (a) – tocopherols, (b) – tocotrienols
Vitamins E in room temperature are oily, water insoluble compounds, easily soluble in fats. In the oxygen free environment. In the oxygen free environment they are thermally stable (up to 200°C) and resistant to the action of acids and bases. However they are sensitive to the UV light and oxygen. In the presence of iron salts they undergo oxidation forming dimers, trimers and quinones. Their sensitivity increases while the number of methyl groups in the chemical structure increase. Therefore while ingesting iron vitamin E shall not be used.
It was also found that vitamin E is responsible for proper function of reproductive system in many species of animals. However till now, no negative symptoms in humans were observed due to a lack of this vitamin. Vitamin E has a positive impact on the red blood cells development in children with anaemia.
It is believed that daily limit for humans fall in the range from 10 to 30 mg of α-tocopherol. The content of vitamin E in nutrition tables is expressed as α-tocopherol equivalents given in mg. Since pure α-tocopherol exhibit 100% of bioactivity, therefore:
1 mg of α-tocopherol equivalent = 1 mg of pure a-tocopherol
In practice both natural and synthetic tocopherols are used. Concentrates of vitamins E are obtained from plant oils (mixture of various tocopherols with majority of α-tocopherol). A very rich source of this vitamin are plant oils, especially from wheat germ, soybean and cotton seeds, as well as lettuce, spinach, cabbage, butter and eggs.
High performance liquid chromatography (HPLC)
Liquid chromatography is an analytical method used for separation of mixtures of compounds. Analytes are separated due to their different physical and chemical interactions between mobile and stationary phase. Analyzed sample is firstly diluted in a selected solvent (frequently in the same as a mobile phase) according to its physicochemical properties and applied separation system. Then diluted sample is injected onto column filled with porous or gel packing. Compounds with a higher affinity to the stationary phase are selectively retained on the column and move slower than compounds with a lower affinity. In this way separation occurs and it is possible to isolate, identify and analyze one compound even from a very complicated mixture. Mobile phase constitute of appropriately chosen solvent (eluent). Stationary phase is chosen according to its polarity. In the earlier times so called “normal phase” chromatography was used, meaning that the stationary phase is far more polar than mobile phase (e.g. silica gel – hexane). Recently much more popular are so called “reversed phases” where mobile phase is usually made of silica gel but chemically modified on the surface with hydrocarbon moiety and mobile phase consist of water mixed in different proportions with medium polar solvent (e.g. octadecyl-silica gel – water : acetonitrile or water : methanol). The most popular detectors used in HPLC are UV-Vis spectrophotometers. Since vitamin E contains chromophore moiety in its chemical structure (it is able to absorb radiation at the given wavelength) this detector is also used in the analysis of thereof. The measure of the chromatographic signal is represented by chromatographic peak, area (or height) of which corresponds to a quantity of an analyzed compound.
In the case of fat soluble vitamins before final HPLC analysis it is frequently necessary to saponify analyzed material.
Reagents
- methanol (HPLC grade)
- acetonitrile (HPLC grade)
- deionized water,
- anhydrous sodium sulfate
- phenolphthalein
- ethanol (98%)
- 0.5M solution of KOH in ethanol,
- petroleum ether p.a.
- α-tocopherol
Hazards
compound |
R and S phrases |
Pictograms |
Acetonitrile |
R: 11; R:20/21/22-36
S: 1/2-16-36/37
|
 
|
Methanol |
R: 11-23/24/25-39/23/24/25
S: (1/2) 7-16-36/37-45
|
 
|
Ethanol |
R: 11
S: 7, S: 16
|

|
0.5M solution of KOH in ethanol |
R: 11
S: (1/2)-7-16-26-36/37/39-45
|

|
phenolphthalein
|
R:40
S: 36/37
|

|
Petroleum ether
|
R: 45-65
S: 53-45
|
 
|
a-tocopherol |
It is considered as hazardous chemical but use of safety phrases and symbols are not obligatory |
|
Materials
- Separatory funnel (500 ml – 2 pcs.)
- Glass funnel – 4 pcs.
- Florence flask 250 ml – 1 pcs.
- Glass flask with closure - 2 ml 2 pcs.
- Glass syringe 100 µl 1 pc
- Round bottom flask 100 ml – 1pcs.
- Water bath
- Reflux condenser
- Graduated cylinder 50 ml – 2 pcs., 100 ml – 2 pcs.,
- Filter paper
- Membrane filter (0.45 m m)
- Beaker 250 ml, 2 pcs.
- Alumina foil
Procedure
Saponification
Weight to round bottom flask 5 g (±0.01g) of sunflower oil. Wrap the flask with alumina foil in order to protect it from the light. Add ca. 1 – 2 g of ascorbic acid and 60 ml of 0.5 M solution of KOH in ethanol. Mount the reflux condenser and place the flask in the water bath (ca. 95°C). Run saponification reaction for 45 min. Decant obtained solution to the separatory funnel. Wash the flask with ethanol (3 times with 5 ml) and add it also to the funnel. Finally, add deionized water to reach the ethanol : water ratio of 2:1 v:v.
Extraction of vitamin E with petroleum ether
Extract the vitamin E with 70 ml of petroleum ether. Move the layer of the extract to the second funnel and extract ethanolic solution twice, each time using 50 ml of petroleum ether. Collect all the extracts gently, not letting to form suspension. Wash the extract with water (4 times with 50 ml) until water remains colorless after addition of 1% phenolphthalein. Remove the traces of water with sodium sulfate. Then evaporate it to dryness onto rotavapor (30°C). Dissolve the residues in 2 ml of acetonitrile. Move two portions (1 ml each) of the solution to two flasks with closure. Analyze one of it directly with HPLC (3 repetitions). To the second one add 20μl of vitamin E solution (1.56 mg/ml) and analyze with HPLC (3 repetitions).
HPLC conditions
HPLC column C-18, mobile phase flow: 0.8 ml/min. wavelength l=292 nm, mobile phase acetronitrile/methanol 80:20 v:v.
Results
Measure the area of the signal given by vitamin E in chromatograms with and without addition of the standard. Place obtained results in the Table 1. Calculate the content of vitamin E using equations 1 and 2.
Table 1. HPLC results
Peak area |
Measurement 1
|
Measurement 2 |
Measurement 3 |
Mean area |
Without addition of the standard |
|
|
|
|
With addition of the standard |
|
|
|
|
c = Y0· cs / ( Yi – Y0) |
(1) |
where:
Y0– peak area for the vitamin E in the sample without addition of the standard,
Yi– peak area for the vitamin E in the sample with addition of the standard,
c –concentration of vitamin E in the sample
cs – concentration of vitamin E in the standard
The concentration of the added standard (cs) is calculated as follows:
where:
Vp– volume of the sample,
cst – concentration of the standard,
Vst – volume of the standard solution added to the sample
Prepared
by: Faculty of Chemistry, University ob Gdansk, Poland
|