|

Isolation and identification of selected plant dyes
The aim
This exercise aims for isolation of dyes extracted from the plant material (dried paprika, parsley), and their further separation and identification by means of thin layer chromatography (TLC).
Introduction
Dyes (pigments) are defined as various chemical entities that are able to absorb electromagnetic radiation in the visible range (400 to 780 nm). Their presence is responsible for specific colors of plants, animals and other objects. There are two main groups of dyes: natural and artificial. Natural dyes are present in fruits, vegetables or edible plant leaves (e.g. carotene, chlorophyll, anthocyanin) as well as in animal tissues (e.g. myoglobin, hemoglobin). Artificial (synthetic) dyes are those not present in the environment, synthesized by man (e.g. quinoline yellow).
In food production often nature – identical colorants are used. These compounds poses chemical structure identical to this found in nature, however they are man-made.
Natural dyes are divided into two general groups according to their chemical structures:
- Carotenoids – isoprene dyes, chlorophylls, myoglobin, hemoglobin
- Anthocyans - flavonoids and others
Isoprene dyes are derivatives of branched hydrocarbons containing 40 carbon atoms (so called tetraterpenes). Their structure is characterized by many conjugated double bonds (polyenes) near always with trans configuration. This is the main factor responsible for intensive color of these substances. Particular compounds differ from each other in the level of hydrogenation, presence of cyclic forms at the both ends of hydrocarbon chain, presence of oxygen in the structure (xanthophylls) or its absence (carotenes). The may be acyclic (lycopene), monocyclic (γ-carotene) or bicyclic (α-carotene and β-carotene). The color of carotenoid dyes vary from yellow, through orange to red. They are present in flowers, seeds, fruits, roots and leaves.
Carotenoids are one of the most effective natural scavengers of singlet oxygen, thus having antioxidant potency and specific biological activity. Carotenoids with b-ring are characterized with pro-vitamin A activity; the highest activity is represented by b-carotene because it contains two b-rings on both ends of the carbon chain.
Chemical structure of selected carotenoids.
The principal process enabling isolation of organic substances from plant material is solid – liquid extraction. Extraction allows for selective transport of substances from solid to the solvent phase. The yield of extraction depends mainly on solubility of substances in particular solvent. Frequently extraction is run in continuous process in the Soxhlet apparatus (see below).
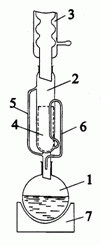
Soxhlet apparatus: round bottom flask (1), extractor (2), thimble (4), condenser (3), distillation path (5), siphon top (6), heating jacket (7).
Frequently, isolated mixture of dyes is separated to particular compounds by means of chromatographic methods. Both planar and column liquid chromatography is used. In planar chromatography (thin layer chromatography TLC) mobile phase form a flat surface. A stationary phase is made of a paper or thin layer of sorbents previously bonded on glass or alumina plates.
In TLC separated analytes are moving together with a solvent (mobile phase) that penetrates a stationary phase. In this course analytes undergo dynamic processes of adsorption, desorption and partition between both phases. All these processes cause that various substances migrate through the system with various velocities finally being separated from each other.
Analyzed substances present in solution are placed onto the TLC plate with a glass capillary in the certain distance from the plate edge. Then plate is placed in the closed chamber filled with a hint of solvent. Solvent is drawn up the plate through the capillary action thus cause migration of analytes through the stationary phase. This process is called development of chromatogram. While solvent front reach the end, the plate is removed from a chamber then it is dried and analyzed. If analyzed compounds are colorless, visualization of the developed chromatogram is made under UV lamp.
In TLC the value that characterize analyzed compound is called retardation factor Rf which d escribes the ratio of time spent in the stationary phase relative to time spent in the mobile phase
Rf = migration distance of substance / migration distance of solvent front
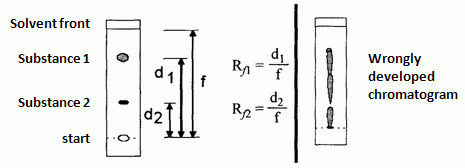
Exemplary TLC chromatogram of 2 analytes mixtures a) properly developed chromatogram, b) wrongly developed chromatogram
(www.chemia.uj.edu.pl/dydaktyka/biofiz2009.pdf).
The Rf value may vary from 0 to 1. If Rf equals 0 it means, that chromatographed substance is too strongly adsorbed in the stationary phase. Whereas if Rf equals 1 it means that substance is not retained and migrates only within the mobile phase. The optimal range of Rf values is between 0.2 and 0.8. Rf depends on type of the analyte, type of mobile and stationary phases, saturation of the chamber with the solvent and temperature.
Reagents
- Dried paprika
- Parsley
- Chloroform p.a.
- Acetone p.a.
- Petroleum ether p.a.
- n-hexane p.a.
- 10% solution of NaCl
- CaCO3 p.a.
- Anhydrous sodium sulfate p.a.
- Silica gel plates (e.g. Silica Gel 60 Merck)
- E 160c (contains capsanthin and capsorubin)
- E 160 a(ii) (contains β-carotene)
Hazards
compound |
R and S phrases |
Pictograms |
Acetone |
R: 36-66-67
S: (2-)9-16-26
|
 
|
Chloroform
|
R: 22-38-40-48/20/22
S: (2-)36/37
|

|
Petroleum ether
|
R: 45-65
S: 53-45
|
 
|
n-hexane
|
R: 38-48/20-51/53-62-65-67
S: (2-)9-16-29-33-36/37-61-62
|
  
|
Sodium carbonate |
S: 22 |
|
Materials and methods
- Analytical balance
- Soxhlet apparatus (flask 250 ml)
- Rotavapor
- Graduated cylinder 200 ml – 1 pc., 50 ml – 3 pcs.
- Glass funnel – 2 pcs.
- Ceramic mortar
- Erlenmeyer flask 50 ml – 2 pcs.
- Filter paper
- Separatory funnel
- Florence flask 50 ml – 1 pc., 100 ml with closure – 1 pc.
- Tubular glass column with stopcock, inner diameter 1 cm, minimal length 15 cm
- Cotton wool
Procedure
Extraction of paprika dyes
Weight 10 g of dried paprika in the extraction thimble and close it with a cotton wool. Place the thimble in the Soxhlet apparatus. Place few boiling chips in the round bottom flask and put 120 ml of chloroform. Place the flask in the heating jacket, connect it with the Soxhlet apparatus, start the cooling water and turn on the heater. Keep the state of mild boiling for 1.5 – 2 hours. Turn off the heating and transfer the extract to the Florence flask. Using rotavapor concentrate the extract to the 10% of the initial volume.
Extraction of parsley dyes
Rub 5g of parsley leaves in the mortar adding 20 ml of the mixture acetone : petroleum ether (22:3, v:v). While rubbing add additionally 0.5 g of calcium carbonate. Filter it through the filter paper and collect the filtrate in the separatory funnel. Add 20 ml of petroleum ether and 20 ml of 10% NaCl solution, then shake vigorously for 2-3 min. After separation of both phases, remove the bottom one and wash the upper phase for 3 times with 5 ml portions of distilled water. Transfer washed extract to the Erlenmeyer flask and add anhydrous Na2SO4 in order to remove residues of water. Further, transfer the extract to the Florence flask and concentrate it on the rotavapor to 20-30% of the initial volume.
TLC analysis
Add a hint of the solvent mixture of n-hexane and acetone (7:3, v:v) to the chamber, close it and condition the system for 15 min. Cut of a piece of TLC plate (ca. 4.5 cm x 8.5 cm), mark with a pencil a start line, and points of samples insertion: point 1 and 3 (standards of dyes), point 2 (paprika extract), point 4 (parsley extract). Place all solutions with a glass capillary on the plate (1-2 drops) and dry it with a warm air from the hairdryer. The diameter of the spots should not exceed 5 mm. Place the plate in the chamber in the way that solvent will be below the start line. After development of chromatogram mark with the pencil the solvent front, evaporate the solvent with the warm air and calculate the Rf values of chromatographed dyes. On the basis of Rf values identify isolated compounds.
Prepared
by: Faculty of Chemistry, University ob Gdansk, Poland
|