|
Developing applications for the SpektraTM spectrometerThis section aims at helping potential users of the SpektraTM spectrometer who are starting to develop their own applications. The differences between general-purpose spectrometers and the SpektraTM spectrometer are indicated in this document, and hints are given, suggesting how to start developing applications that will be successful in this process. The SpektraTM spectrometer is not a replacement for a general-purpose spectrometer. Both have their own specific purpose and focus. Achieving the highest possible accuracy and precision of an absorbance measurement is the main objective with general-purpose spectrometers. The wavelength selection has to be as accurate as possible and the light highly monochromatic. As a consequence, the construction is complicated and not very obvious to a typical user. Buying a general-purpose spectrometer is quite an investment. The objective when developing and designing the SpektraTM spectrometer was different. The main aim was to produce a low-cost, portable and robust instrument with a simplified and easily understandable design and operation, which allows for low reagent consumption and a simplified experimental approach. The SpektraTM is an empirical instrument, primarily intended for educational purposes, especially for the introduction of concepts, but it has also proved useful for on-the-spot quantitative or semi-quantitative determinations of the different parameters of real samples. Not every application performed on a general-purpose spectrometer is transferable to the SpektraTM, and most applications that are transferable will need at least some modification for a successful outcome. What are the limitations in the transferability of applications, and what has to be taken into account? There are three main aspects:
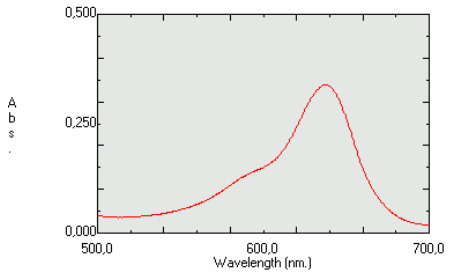
Consideration of the shape of the absorption spectrum of the coloured product in relation to the emission spectrum of the light sourceThe suitability of an application for the SpektraTM spectrometer has to be considered first. The SpektraTM has three light emitters, blue, green and red, with nominal wavelengths of 430 nm, 565 nm and 625 nm, respectively (to observe the shape of the emission spectra of the light emitters please click on the related links). Developing an application for the SpektraTM can only be successful if the coloured product in solution can effectively absorb the light of a selected light source. This can only happen if the absorption spectrum of the coloured product in the solution being examined overlaps significantly with the emission spectrum of the light source. Therefore, the wavelengths of the absorption maximum of the coloured product and the wavelength of the emission maximum of the selected light emitter must not be too different, and it is preferable to have wide absorption peaks with flat tops. Such a situation is illustrated with an example. Example: We would like to find out if the SpektraTM spectrometer is suitable for determining a green dye in a Mint natural aroma (Tovarna arom in eteričnih olj, d.d., Slovenia). A spectrum of the Mint aroma was recorded with a general-purpose spectrometer (Picture 1). A comparison of the shape of the absorption spectrum of the Mint aroma and the emission spectra of the three light emitters indicated that the red light emitter (red LED) is expected to be appropriate for this application.
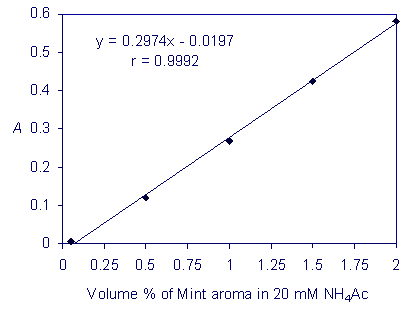
Picture 1. Absorption spectrum of a solution of the Mint natural aroma (0.16 volume %) in the ammonium acetate solution, 20 mmol/L The suitability of this application for the SpektraTM was further tested by preparing a series of 11 solutions of the Mint aroma in an ammonium acetate solution, 20 mmol/L. The concentrations of the Mint aroma solutions extended from 0.05 to 5 volume %. 450 ml of each solution was transferred into the hollows of a blister. The transmittances of the solutions were measured with the SpektraTM using the red LED against an ammonium acetate solution, used as a blank. The transmittances were transformed into absorbances. The absorbances were then plotted on a graph against the Mint aroma solution concentrations. It was confirmed that the absorbance is linearly related to the Mint aroma solutions’ concentrations in the concentration range extending from 0.05 to 2 volume %, as shown in Picture 2, with a high correlation coefficient of 0.9992. The experiments proved that it is possible for the green dye in the Mint aroma to be quantitatively determined with the SpektraTM spectrometer using the red LED.
Picture 2. Calibration line
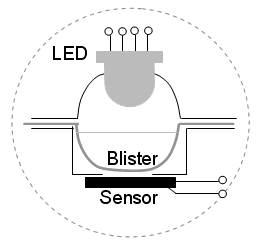
for Mint aroma Liquid HandlingDue to the specific, vertical optical geometry of the measuring chamber in the SpektraTM spectrometer (Picture 3) the liquid layer in the hollow of a blister determines the light’s path length through the examined solution. Therefore, equal liquid layers of the solutions in all the hollows of a blister within the same experiment are essential.
Picture 3. Optical geometry of the measuring chamber of the SpektraTM spectrometer Proper liquid handling is very important, but different approaches can be used. The approach most frequently used by new users of the SpektraTM is preparing solutions in a classical way, e.g., in a volumetric flask and transferring the equal volumes of solutions into the hollows of a blister with a micropipette. Another, better and more green-chemistry-oriented way of using the SpektraTM spectrometer is to prepare the examined solutions directly in the hollows of a blister, and consequently use less chemicals and produce smaller quantities of chemical waste. But care has to be taken that all the additions by micropipettes in each hollow of a blister sum up to the same total volume, presuming the volumes are additive. This approach reduces the number of operations spent in liquid handling and makes the procedure faster. But the question of adequate mixing may arise. Due to the small volume of the hollow of a blister and its concave shape, efficient homogenisation of the solution is usually rapidly achieved by moving the blister gently along the surface of a laboratory desk for a short period of time. A third possible approach, suitable for semi-quantitative analysis or the introduction of concepts in schools, is preparing the liquids in the hollows of a blister with a drop-based approach. In this approach ten-millilitre polymeric dropping bottles are used for the liquid handling, and a drop is considered as a volume unit. The total number of added liquid drops must be equal for all the hollows of a blister. However, there are different sources of uncertainty in this approach that a potential user has to consider. The most important are the limited repeatability of the drop volumes dispensed by the dropping bottles and the effect of the differences in viscosity and the surface tension of the solutions on the volume of the drops dispensed. The repeatability of the drop volumes strongly depends on the experimental technique. All the dropping bottles must be of the same kind; dropping tubes are by no means suitable. The dropping bottles must not be either too full or too empty, since this would affect the volume of a drop. The best way is to fill the bottle to three-quarters of its volume. The correct experimental technique is demonstrated in Picture 4.
Picture 4. Dispensing drops into the hollow of a blister using a dropping bottle It is advisable that the first drop is dispensed into a beaker. In this way it is possible to check if the drop has been formed correctly, without an air bubble. During the dispensing of liquid a bottle has to be in its vertical position. During its formation a drop must not be allowed to come into contact with the liquid surface in the hollow of a blister; otherwise its volume will be lower. A drop must fall off the dropper tip spontaneously; therefore, it is advisable that the hand is supported, to prevent shaking. The repeatability of the drops’ formation was evaluated by weighing. The experiment proved that a repeatability with a relative standard deviation (RSD) of 8% is easily achievable, but a RSD as good as 3% can be achieved by a well-skilled person with some designs of dropping bottles. It is possible to be sceptical about using a drop-based experimental approach, but experiments proved that calibration lines with correlation coefficients as high as 0.99 can be obtained, and the reproducibility of the procedure is not necessarily as bad as might be expected. Two examples of a calibration line for determining the calcium in water samples obtained with a drop-based experimental approach are demonstrated in Picture 5. These two lines were obtained by two students of chemical education, Mojca Vrtič and Metka Srebotnik, who participated in the optimisation of the analytical procedures for water analysis with the SpektraTM during their diploma work in 2001 and 2002, respectively. Picture 5. Reproducibility of a calibration line for the spectrometric determination of calcium in water with a drop-based experimental approach and the SpektraTM There are several procedures on the web pages of
the Project for which a drop-based experimental approach
was used. If someone prefers to use micropipettes, the
procedures can be easily accommodated to take into account
that the volume equivalent of a drop is between 36 and
45 mL for the majority of
dropping bottles available, depending on their design.
The most important factor for an optimised procedure
is that the proportions of reagents and sample remain
unchanged, and of course it is better to have a thicker
liquid layer in the hollow of a blister for a higher
sensitivity of the procedure. At the same time, care
has to be taken that the hollow of a blister is not
too full, thereby preventing the LED from coming into
contact with the solution and causing spilling after
closing the cover of the measuring chamber of the SpektraTM. Modification or optimisation of the procedure with the objective of increasing its sensitivityIf the transferability of an application from a general-purpose spectrometer to the SpektraTM spectrometer is confirmed, a problem with significantly lower absorbances than in the original procedure may arise. There are two reasons for lower sensitivity. The first reason is that the light’s path length through the examined solution is shorter - approximately 0.3 cm long - in the SpektraTM than in conventional spectrometers using a 1-cm optical cell. The second reason lies in the fact that the light emitted by an LED is far less monochromatic than the light from a monochromator in conventional spectrometers. If the absorbances obtained with the SpekteraTM prove to be too low, some modification to the procedure can help out. Approaches contributing to an increased sensitivity, demonstrated by a steeper calibration line are as follows: lowering the sample dilution, increasing the colour-forming reagent concentration, optimising the volume proportions between the reagents and the sample, and increasing the liquid layer in the hollow of a blister to its acceptable maximum. With this approach a significant improvement in the sensitivity of most procedures can be observed, and students challenged to achieve this, e.g., during their project work, can learn a lot during the process.
|