|

A
SIMPLIFIED GAS CHROMATOGRAPH
Hazards
|

|
The
carrier gas is a
mixture of 40 %
propane and 60 %
butane. The mixture
is a highly flammable
liquefied gas and
is kept under pressure
in a gas-supply
cylinder. The cylinder
must not be exposed
to direct sunlight
or temperatures
over 50ºC. It must
be kept in a well-vented
area.
R:12
S:(2-)9-16-33,
|
|

|
Dichloromethane
and trichloromethane
irritate the eyes
and skin, cause
dizziness, nausea,
and headache; they
are also both potential
occupational carcinogens.
Poisonous gas is
produced in a flame.
Even in very small
amounts, careful
handling is required.
Wear suitable protective
clothing and gloves.
R:
40 S:(2-)23-24/25-36/37
R:22-38-40-48/20/22
S:(2-)36/37
|
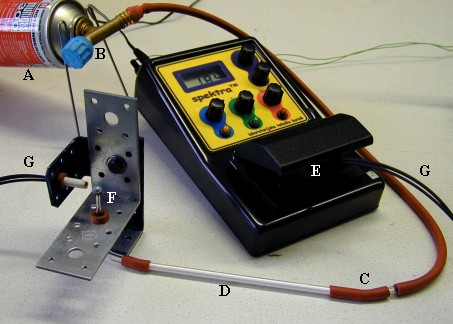
Assembling
the gas chromatograph
For
a hands-on introduction to the
fundamentals of gas chromatography,
a simplified gas chromatograph
for the separation and determination
of chlorinated hydrocarbons
can be assembled in a school
laboratory, as demonstrated
below. The chromatograph consists
of the following: a carrier-gas
supply (A), which is a mixture
of 40 % propane and 60 % butane;
the flow regulator (B); the
injection port (C), and chromatographic
column (D), packed, in our case,
with CALGON® anti-limescale
powder. The SpektraTM spectrometer
functions as a detector (E).
The
detection of chlorinated hydrocarbons
is based on the Beilstein reaction.
In this test a bluish-green
light is emitted when substances
containing chlorine come into
contact with red-hot heated
copper wire (F). The light from
the Beilstein detector is transmitted
to a photoresistor of the SPEKTRATM
spectrometer through an optical
fiber (G). In order to prevent
interference from the surrounding
light, a black, easily removable
light-protection shield is placed
over the optical set-up (not
shown in the picture).
Experiments
with the simplified gas chromatograph
The
simplified gas chromatograph
enables students to obtain hands-on
experience in gas chromatography.
Different experiments can be
carried out, which provide students
with an easy transition from
visual observations of the separation
of a vapour mixture, to a measuring
process and the introduction
of the basic chromatographic
parameters.
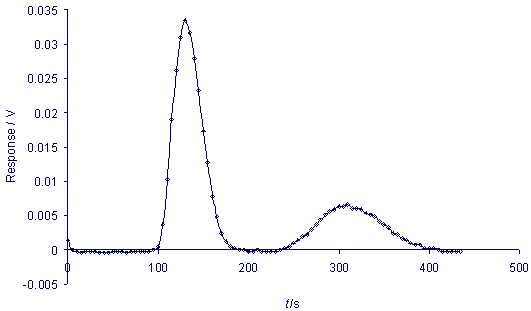
If
a 200 µl sample of a vapour
phase that is a mixture of dichloromethane
and thrichloromethane (chloroform)
is injected into the chromatograph,
a chromatogram similar to the
one presented in the picture
below can be obtained. If
two students work in a pair
they can easily follow the time
on a stopwatch and read measurements
from the spectrometer at five-second
intervals. The first peak relates
to dichloromethane and the second
to trichloromethane. Excel software
can be used to present the measurement
data. The fundamental chromatographic
parameters, e.g., retention
time, peak height, and peak
area, can then be easily introduced.
If
a computerised data-acquisition
and measurement procedure is
preferred, the analogue output
of the SpektraTM spectrometer
can be used.
Developed
and prepared
by:
Nataša
Gros, University of Ljubljana,
Faculty of Chemistry and Chemical
Technology and Margareta Vrtačnik,
University of Ljubljana, Faculty
of Natural Science and Engineering
|