|

A
SIMPLIFIED GAS CHROMATOGRAPH
Transfer
into school
Gas chromatography is frequently
used in food analysis for determining
volatile components of food.
Students of Srednja agroživilska
šola Ljubljana take Analytical
chemistry course (FOOD Analysis)
in the final year of their professional
education. School has no professional
analytical instruments. Simplified
analytical instruments mean
a great help to teachers since
they help students understand
the aims of the learning process.
Our version of gas chromatograph,
assembled following the instructions,
is presented in Pictures 1 and
2. The most difficult for us
was filling a chromatographic
column properly. If the column
was filled too tight, the carrier
gas did not flow through the
column. If the column was filled
too lose the carrier gas flew
through the column to fast and
the separation did not take
took.

Picture 1. A simplified gas
chromatograph assembled at Srednja
agroživilska šola Ljubljana.

Picture 2. Optic fibre and
Beilstein burner of a simplified
gas chromatograph.
The operation of gas chromatograph
was tasted by injecting 300
ml
of a vapour phase of a mixture
of dichloromethane and trichloromethane.
At the beginning we observed
the blue-green light, related
to the elution of the two sample
components and Beilstein reaction
in flame of a burner. Afterwards
measurements were taken during
elution and repeated three times.
Results we obtained are
summarised in Table 1 and presented
graphically in Picture 3. Peaks
are separated, the first peak
relates to dichloromethane and
the second to trichloromethane.
Experiments help students understand
separation process and chromatographic
parameters, e.g., retention
time, peak area.
|
t/s |
Response |
|
5 |
0.026 |
|
10 |
0.024 |
|
15 |
0.024 |
|
20 |
0.024 |
|
25 |
0.024 |
|
30 |
0.025 |
|
35 |
0.039 |
|
40 |
0.039 |
|
45 |
0.04 |
|
50 |
0.044 |
|
55 |
0.049 |
|
60 |
0.047 |
|
|
t/s |
Response |
|
65 |
0.044 |
|
70 |
0.036 |
|
75 |
0.024 |
|
80 |
0.025 |
|
85 |
0.045 |
|
90 |
0.047 |
|
95 |
0.048 |
|
100 |
0.047 |
|
105 |
0.047 |
|
110 |
0.047 |
|
115 |
0.043 |
|
120 |
0.033 |
|
|
t/s |
Response |
|
125 |
0.03 |
|
130 |
0.031 |
|
135 |
0.032 |
|
140 |
0.031 |
|
145 |
0.032 |
|
150 |
0.029 |
|
155 |
0.03 |
|
160 |
0.031 |
|
165 |
0.03 |
|
170 |
0.03 |
|
175 |
0.029 |
|
180 |
0.03 |
|
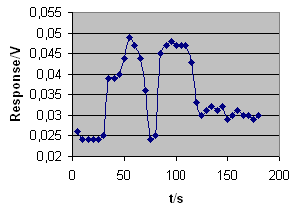
Picture 3.
Chromatogram of a vapour mixture
of dichloromethane and trichloromethane
Prepared by: Alma
Kapun Dolinar, Srednja agroživilska
šola, Ljubljana and Irena Štrumbelj
Drusany, Srednja agroživilska
šola
|