|

5. A COLORIMETRIC DETERMINATION OF
NITRITE
IN A WATER SAMPLE
5.1 Objective
Estimate the concentration of nitrite
ions in a water sample. Perform the procedure
as indicated below and compare the final
coloration of the examined solution with
the coloration of the three standards, which
have nitrite concentrations of 0.01 mg/L,
0.1 mg/L and 0.5 mg/L.
5.2 Background
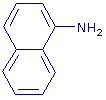
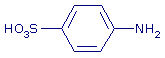 + NO2- + 2 H+ + NO2- + 2 H+  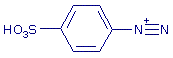 + 2 H2O + 2 H2O
 + +
 + H+ + H+
Red coloration
5.3 Equipment and reagents*
- A solution of sulfanilic acid.
- A solution of 1-naphthylamine
- An acetate buffer solution of pH
3.03, concentration 1.58 mol/L.
- Three standard solutions of NaNO2
with different concentrations.
*For reagent preparation please contact
natasa.gros@fkkt.uni-lj.si.
Hazards
|

|
Sulfanilic acid (4-aminobenzenesulfonic
acid ) causes skin and
respiratory-tract irritation.
It can also cause an allergic
reaction.
R 36/38-43, S 24-37
|
|

|
1-naphthylamine can
effect the central nervous
system.
R: 45-22-51/53, S: 53-24-45-61
|
|
|
Acetic acid is corrosive.
Prolonged or repeated skin contact
can cause dermatitis.
R: 10-35, S: 23-26-45
|
5.4 Procedure
For the blank, all three standards and
the sample, follow the procedure as given
below.
| |
Blank |
Standard |
Sample |
|
Deionised water |
4 S
|
/ |
/ |
|
Standard solution of nitrite |
/ |
4 S
|
/ |
|
Sample |
/ |
/ |
4 S
|
|
Sulfanilic acid |
1 S
|
1 S
|
1 S
|
|
Mix thoroughly, wait five minutes. |
|
1-naftilamin |
1 S
|
1 S
|
1 S
|
|
Buffer solution |
3 S
|
3 S
|
3 S
|
6
Mix thoroughly, after ten minutes estimate the concentration of nitrite
ions in the sample solution.
?
Write the result of the estimation in the table “Results – Contaminants”,
under the serial number of the sample.
The maximum admissible concentration
(MAC value) for nitrite in drinking water
which is set by the EU Drinking Water Directive
(Council Directive 98/83/EC) is 0.5 mg/L.
Note:
If you prefer to do the experiment
with a spectrometer, use the nitrite standard
with the highest concentration for the one-point
calibration. Measure after 10 minutes, use
the green LED.
| |
T [%] |
A |
gNO2- [mg/L]
|
|
Standard |
|
|
0.5 |
|
Sample |
|
|
|
|