|
Thiocyanate is one of the
oldest reagents for determination
of iron (III) ions in different
samples. It is neither very
sensitive nor very selective.
We selected this reagent, since
it is readily available. EQUIPMENT AND REAGENTS
HAZARDS
|
||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
|
Hydrochloric acid is corrosive and toxic: it causes severe irritation of the upper respiratory tract and is harmful if absorbed through the skin. Avoid inhalation and skin contact. R: 24/25-34,
S: 28.2-45 |
|
|
Potassium
thiocyanate R: 20/21/22-32,
S 13 |
|
|
Iron(III) chloride hexahydrate causes chemical burns to the respiratory tract. It can also cause skin burns, and when in contact with acids it liberates toxic gases. R: 31-34, S:
28-45-50.1 |
PROCEDURE
Preparation of a condiment sample:
- Weigh a clean and dry crucible.
- Grind and homogenize condiment samples.
- Weigh 1 g of a condiment sample in a crucible.
- With the burner start slowly heating the crucible with the sample. After reduction of the volume, ignite the sample until a white or grey ash with no black particles is obtained (for basil one and a half hour will do, other samples will need more time).
- After completion of the ignition, let the crucible cool down to room temperature.
- Weigh the crucible with the ash sample.
- Add 5 mL of 2 M HCl into the crucible.
- Dry the content of the crucible on a water bath.
- Cool down and add 3 mL of deionised water, 5 mL of 2 M HCl and 5 mL of 0.1 M KSCN.
- Filter the sample solution through a filter paper with fine porosity. Discard the first third of the filtrate and collect the other part in a clean and dry glass vial with a cap.
Preparation of a diluent solution and a stock solution of iron(III) thiocianate complex:
- Prepare a diluent solution by mixing in a dry and clean vial 3 mL of deionising water, 5 mL of 0.1 M KSCN, and 5 mL of 2 M HCl.
- Prepare a stock solution of an iron (III) thiocyanate complex in a dry and clean vial by mixing 0.5 mL of 0.05 M FeCl3 solution with 2.5 mL of deionised water and 5 mL of 0.1 M KSCN solution, and 5 mL of 2 M HCl.
Spectrometric measurement
Prepare a blank, sample(-s) and a series of calibration solutions on a blister as follows:
|
|
g(Fe3+) [mg/L] |
Stock
solution of the
complex |
Diluent
solution |
Sample
solution |
|
Blank |
- |
0 |
450 |
- |
|
Sample |
- |
- |
- |
450 |
|
Calibration solution 1 |
12.1 |
50 |
400 |
- |
|
Calibration solution 2 |
24.3 |
100 |
350 |
- |
|
Calibration solution 3 |
36.4 |
150 |
300 |
- |
|
Calibration solution 4 |
48.6 |
200 |
250 |
- |
|
Calibration solution 5 |
60.7 |
250 |
200 |
- |
|
Calibration solution 6 |
72.9 |
300 |
150 |
- |
|
Calibration solution 7 |
85.0 |
350 |
100 |
- |
|
Calibration solution 8 |
97.2 |
400 |
50 |
- |
|
Calibration solution 9 |
109.3 |
450 |
0 |
- |
Insert a blister into the measuring chamber of a SpektraTM spectrometer so that a blank solution occupies the measuring site. Use blue LED at its maximal intensity and set the transmittance for the blank to 100.0. Take measurements for samples and calibration solutions and write the transmittances into the table below. Calculate absorbance and prepare a calibration graph as demonstrated below.
|
|
|
Calibration solutions |
||||||||
|
|
Sample |
1 |
2 |
3 |
4 |
5 |
6 |
7 |
8 |
9 |
g(Fe3+) [mg/L] |
? |
12.1 |
24.3 |
36.4 |
48.6 |
60.7 |
72.9 |
85.0 |
97.2 |
109.3 |
T |
|
|
|
|
|
|
|
|
|
|
A |
|
|
|
|
|
|
|
|
|
|
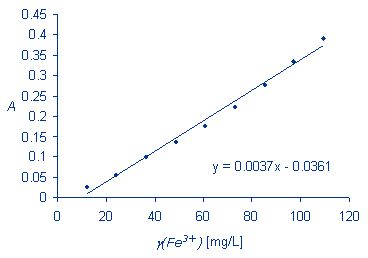
Use the linear equation obtained for your calibration data and calculate the iron concentration in the sample solution.
Note
If the absorbance of the sample solution exceeds the absorbance of the final calibration solution, the sample solution must by no means be diluted with water. This would shift the equilibrium and consequently cause erroneous results. The sample can only be diluted with a diluent solution. Dilution must be taken into account while calculating the iron content in the condiment sample.
The iron content per 100 g of the condiment sample is then calculated as follows:
|
m(Fe3+) = |
g(Fe3+) · 0.013 L · 100 g |
|
msample |
Worked out example
In our case 1.0041 g of a dry basil sample was taken for analysis. The transmittance of a sample solution measured against the blank using the blue LED was 77.5.
A = -log(77.5/100) = 0.1107
0.1107 = 0.0037 x - 0.0361
g(Fe3+) = x = 39.67 mg/L
|
m(Fe3+) = |
39.67 mg · 0.013 L · 100 g |
= 51.4 mg |
|
L · 1.0041 g |
The iron content in a dry basil sample is 51.4 mg per 100 g of the condiment.
Developed
and prepared
by: Margareta
Vrtačnik*, Vida Mesec*, Nataša
Gros** and Domen Klančar**
*University of Ljubljana, Faculty of Natural Sciences
and Engineering
**University
of Ljubljana, Faculty of Chemistry
and Chemical Technology
